Zinc spray metallizing
Zinc spraying, or metallizing, is accomplished by feeding zinc powder or wire into a heated gun where it is melted and sprayed onto the steel component using combustion gases and/or auxiliary compressed air. There are automated metallizing processes, but often the coating is still applied by a skilled operator. Prior to metallizing, the steel must be abrasively cleaned to a near white metal. As the steel surface must remain free of oxides, it is common to clean smaller areas, coat the area, and then move to the next section of the part—adding time and cost to the coating process. The coating is 100 percent zinc and is often sealed with a low-viscosity polyurethane, epoxy-phenolic, epoxy, or vinyl resin. Metallizing is most commonly shop-applied, but can also be done in the field.
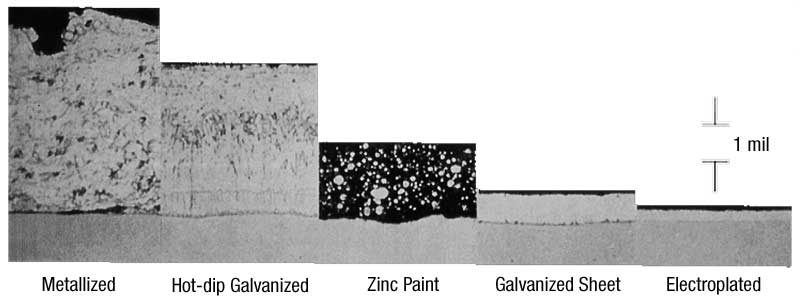
The metallized coating is rough and slightly porous. However, as it is exposed to the atmosphere, zinc corrosion products tend to fill the pores, providing consistent cathodic protection. The metallized coating can be applied to meet any thickness requirements (with a density of approximately 80 percent of hot-dip galvanizing), but can have adhesion issues if it is excessively thick. The metallized coating cannot be applied to interior surfaces and tends to thin at corners and edges, which are harder to achieve consistent spray thicknesses than flat surfaces.
Zinc spray can be applied to materials of any size, and is often used as an alternative to, or in conjunction with, batch hot-dip galvanizing when a part is too large for immersion in the galvanizing kettle. Metallized coatings are increasing in popularity in the bridge market, as many counties and Departments of Transportation (DOTs) look to meet a 100-year service life for their bridges. Although the coating is more commonly applied in the shop, it is possible to apply in the field, making it a great option for extending the life of a steel structure already in place. The biggest limitations to metallizing applications are availability (skilled operator and/or equipment) and the cost premium.
Zinc-rich paints
Zinc painting, often erroneously called ‘cold galvanizing,’ is the brush or spray application of zinc dust mixed with organic or inorganic binders. Zinc-rich paints typically contain 92 to 95 percent metallic zinc in dry film, and are often coated with other intermediate and/or topcoat paint systems. Prior to the paint application, the steel is blasted to a near white metal (The Society for Protective Coatings Standard SSPC-SP10) or white metal (SSPC-SP5). The zinc dust is mixed with a polymeric-containing vehicle and is constantly agitated during application to ensure a homogenous mixture of zinc and proper adhesion. Zinc-rich paints can be applied in both the shop and the field (if the environmental conditions meet the paint manufacturer’s requirements).
Zinc-rich paint, like all paints, is a surface coating mechanically bonded to the steel at a few hundred pounds per square inch (psi). Both organic and inorganic paints can be applied to varying levels, but potential for cracking increases with thickness. There are some performance differences between inorganic and organic zinc-rich paints. Inorganic formulations can withstand temperatures up to about 371 C (700 F), and do not chalk, peel, or blister readily. Inorganic zinc-rich paints, which have about half the density of zinc per mil as batch hot-dip galvanizing, are easy to weld and provide simpler cleanup than organics. The properties of organic zinc-rich paints depend on the solvent system. Multiple coats can be applied in a short period without cracking. However, organic formulations are limited to temperatures of 93 to 148 C (200 to 300 F) and are subject to ultraviolet (UV) degradation.

Photos © SeanBrecht
Zinc-rich paints can be applied to any size and shape of steel, and are often used as primers to high-performance two- and three-coat systems. They are also widely used for touch-up and repair of hot-dip galvanized coatings. In mild environments, zinc-rich paints can be used independently; however, using them alone in more severe environments may not be advised because the cathodic protection afforded is more limited than other zinc coatings.
Like other zinc coatings, it is possible for zinc-rich paints to provide cathodic protection, but in order to do so, it is critical to have zinc dust in high enough concentrations to provide for conductivity between the zinc particles and the steel. There is some question about the cathodic capabilities when the particles are encapsulated in the binder and the binder is non-conductive.
Continuous sheet galvanizing
Continuous sheet galvanizing is a hot-dip process similar to batch galvanizing, but it is only applied to steel sheet, strip, or wire. It is a coil-to-coil process where steel sheet from 0.2 to 43.8 mm (0.01 to 1.7 in.) thick and up to 1828 mm (72 in.) wide is passed as a continuous ribbon through cleaning baths and the molten zinc at speeds up to 183 m (600 ft) per minute. Prior to coating, the continuous sheet is cleaned, brushed, rinsed, and dried. Then, the steel passes into the heating or annealing furnace to soften it and impart the desired strength and formability. While in this furnace, any oxides on the surface are also removed, so it is clean before dipping in the molten zinc bath. As the sheet is withdrawn from the bath, precisely regulated, high-pressure air (known as an air knife) is used to remove any excess zinc to create a closely controlled coating thickness.




