Avoiding galvanic corrosion with dissimilar metals
by sadia_badhon | August 21, 2020 10:01 am
by Alana Fossa
 [1]
[1]Today’s architects and designers are increasingly looking toward a trendy selection of metals such as stainless steel, aluminum, galvanized steel, painted steel, copper, and weathering steel for commercial building projects. Mixing and matching these metals to achieve a unique combination of colors, textures, patinas, and sheens can add further visual appeal, but such artistic choices require careful consideration when constructing sustainable and low-maintenance exterior structures to last generations. When two different metals are in contact and exposed to moisture containing salts or pollutants, one of the metals can experience accelerated corrosion while the other remains protected. This type of accelerated corrosion between the dissimilar metals negatively impacts the overall corrosion protection of the project and is referred to as galvanic corrosion, bi-metallic corrosion, or dissimilar metal corrosion.
Galvanic corrosion is particularly applicable for projects where hot-dip galvanizing, a process used to apply a metallic coating of zinc and zinc alloys, is specified for the long-term corrosion protection of exterior iron and steel components. Hot-dip galvanized (HDG) steel has a history of providing steel structures with decades of maintenance-free corrosion protection, 100 percent recyclability, low environmental impact, and an industrial aesthetic. These properties continue to make HDG steel a popular and sustainable choice for steel framing, architecturally exposed elements, balcony structures, fencings, railings, fasteners, etc. Since galvanic corrosion can occur at a high rate under certain circumstances, it is important to evaluate the combination of HDG steel with other metals commonly used in building and architecture projects in order to determine whether galvanic corrosion is of concern and how to mitigate it.
 [2]
[2]Galvanic corrosion and the galvanic cell
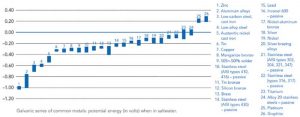
When exposed to a conductive medium such as moisture or saltwater, metals have different electrical potentials. These electrical potentials can be quantified and arranged in order within a particular medium, called a galvanic series. The actual electrical potential values and order of metals vary depending on the specific electrolyte present, but the most common example is the galvanic series of metals in saltwater (Figure 1) (refer ASTM G82-98 [2014], Standard Guide for Development and Use of a Galvanic Series for Predicting Galvanic Corrosion Performance]. Metals such as zinc that is near the top of the galvanic series are more active with a greater negative electrical potential, while metals toward the bottom of the series are more noble or stable. When any two metals of differing potential are combined under specific conditions, a galvanic cell is formed.
In building structures, a galvanic cell (Figure 2) mostly involves direct contact between two different metals exposed to direct moisture or to a humid, marine, or, otherwise, corrosive atmospheric environment. Galvanic cells can also develop upon moisture run-off from one metallic surface to another below. In a galvanic cell, the more active metal gives up electrons to the more noble metal via an electric current. This process results in accelerated corrosion of the more active metal while the more noble metal receiving the electrons is preserved. However, just because dissimilar metals are in contact does not mean galvanic corrosion will occur.
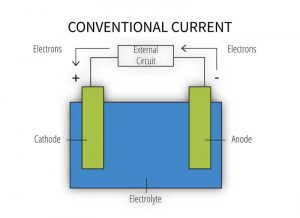 [3]
[3]The formation of a galvanic cell (Figure 2) requires the presence of all the following components:
- anode – metal of greater negative electrical potential, where electrons are generated by the reaction (accelerated corrosion occurs here);
- cathode – metal of less negative electrical potential, where electrons are received (this metal is protected from corrosion);
- electrical connection – such as direct contact between the anode and cathode; and
- electrolyte – conductive medium allowing electrons to be transferred from the anode to the cathode (e.g. exposure to humid atmospheric conditions, moist soil, or common moisture such as water, rain, dew, snow, condensation, or sea spray) (for more information, read Galvanic Corrosion, Metals Handbook [ninth edition] by Robert Baboian).
When any one of these components is absent, galvanic corrosion cannot occur. Similarly, the risk of galvanic corrosion is considered negligible for sheltered conditions such as the interior of climate-controlled buildings, or very low for structures located in arid climates and atmospheric conditions of low corrosivity.
Rate of galvanic corrosion for galvanized steel
If the potential for galvanic corrosion is present on the project due to the use of galvanized steel with other metals, various factors can influence the rate of accelerated corrosion. These factors may or may not result in conditions considered tolerable in service. Zinc tends to be high on the galvanic series of metals in various electrolytes, meaning the HDG coating is anodic to most other metals. The galvanized coating will not only sacrifice itself to protect the underlying base steel, but also try to protect other connected metals such as copper, stainless steel, carbon steel, and aluminum. This will lead to a more rapid consumption of the zinc coating, and decrease the overall longevity afforded. Generally, the corrosion rate increases when combining galvanized steel with metals farther and farther away from zinc in the galvanic series (Figure 3).
 [4]
[4]The aggressiveness of the electrolyte or installation environment can also impact the severity of corrosion. In many atmospheric applications, rainwater and dew are the primary electrolytic materials, but they do not contain many salts and ions, which would make them highly conductive. Alternatively, industrial and marine environments make strong electrolytes because they contain a heavy amount of salts and ions. Even within a single building structure there may be different degrees of exposure to electrolytes of varying strength, such as a building situated near a coastline receiving regular ocean spray to only one face.
However, the corrosion rate is also influenced by the surface area ratio of the different metals. A high zinc corrosion rate can be reduced if the combination of a small area of galvanized steel with a large area of cathodic metal is avoided. Where applicable, a zinc-to-metal surface ratio of at least 10:1 is helpful to minimize the impact of galvanic corrosion on galvanized steel members (read “Opposites Attract: A Primer on Galvanic Corrosion of Dissimilar Metals,” by Christopher Hewitt, Alan Humphries, and Eric Twomey). For example, specifying stainless steel fasteners to join HDG steel framing may be considered, while the use of galvanized fasteners to combine cathodic metals such as carbon steel, copper, or stainless steel should be avoided.
The rate of galvanic corrosion may also be affected by the presence of metal oxide layer formation, the use of corrosion inhibitors, chemical reactions, physical and chemical homogeneity of the metal surfaces, and other environmental effects (temperature, oxygen content, effect of electrode potential, etc.) (for more information, read Galvanic Corrosion, Metals Handbook [ninth edition] by Robert Baboian and also read Galvanic Corrosion of Zinc and It’s Alloys by X.G. Zhang). As galvanic corrosion is a very complex issue, it can be difficult to predict the suitability of the various metals in direct contact with HDG steel. To assist the specifier, the American Galvanizers Association (AGA) offers some guidance (Figure 4) through the interpretation of available corrosion data to predict the impact of galvanic corrosion on HDG coatings based on a limited set of variables (electrical potential, environment, and surface area ratio) (consult Additional Corrosion of Zinc and Zinc Based Alloys Resulting From Contact With Other Metals or Carbon by the British Standard Institute, MIL-STD-889C, Department of Defense Standard Practice: Dissimilar Metals [22-AUG-2016], and American Iron and Steel Institute’s Committee of Stainless Steel Producers, April 1977).
 [5]
[5]In some cases where Figure 4 indicates low or moderate impact on the long-term corrosion resistance of galvanized steel, the galvanic corrosion may be tolerable on a project by project basis. As an example, the Cliffwalk at Capilano Suspension Bridge Park (Figure 5) in Vancouver, British Columbia, Canada, employs a mixture of HDG steel and stainless steel to achieve a light and airy feel to the observation walkways and staircases and platforms. Together in this environment and with favorable zinc-to-stainless surface area ratios, these metals are capable of ensuring decades of maintenance-free corrosion protection in a rainforest-like climate such as Capilano Canyon with a negligible impact on overall longevity. For other projects, the following list elaborates on common combinations where additional measures may or may not be required to ensure longevity of the HDG coating.
Galvanized steel with aluminum or stainless steel
Generally, the combination of HDG steel with stainless steel or aluminum is considered acceptable in atmospheric environments with mild humidity and air salinity. For marine or other high chloride applications, the HDG coating can experience moderate-to-aggressive corrosion and noticeably impact the performance of the galvanizing unless mitigations are put in place.
Galvanized steel and brass/copper
In mild or moderate atmospheric environments, it is preferred to avoid the use of brass/copper materials such as flashings, roofing, or gutters with galvanized components or fasteners to ensure maximum longevity of the galvanized coating. For marine or immersion applications, a fairly severe impact to the galvanized coating performance may occur if connecting these metals without preventative measures.
Galvanized steel and painted carbon steel
 [6]
[6]Galvanized steel, may be successfully combined with painted steel assuming that a durable paint coating system is applied and diligently maintained. Proper maintenance of the paint system is required to ensure full barrier protection is achieved between the galvanized coating and the carbon steel beneath the paint barrier. Where necessary, it is preferable to paint both metals in order to minimize exposure of the galvanizing should the painted components experience damage or weathering.
Galvanized steel and weathering steel
When galvanized steel is combined with weathering steel, zinc will initially sacrifice itself until the weathering steel patina, a protective and passive layer of rust, develops and prevents further sacrificial action. Studies on the use of galvanized fasteners with weathering steel guardrail panels indicate batch HDG fasteners have sufficient zinc thickness to last until the weathering steel patina develops (typically one to five years) with only minimal impact in overall performance. As a result, weathering steel may be joined with galvanized fasteners despite a low zinc-to-metal surface area ratio (read “Atmospheric Corrosion Performance of Hot-Dip Galvanized Bolts for Fastening Weathering Steel Guardrail” by H.E. Townsend, et al).
Galvanized steel and other zinc-coated items
Occasionally, material availability or design requirements result in the specification of multiple zinc coatings within a project. Mechanically galvanized fasteners may be used to connect HDG members in high-strength structural connections, or continuous galvanized sheet products may be joined to batch HDG members with a variety of zinc-coated fasteners. Since the batch HDG coating comprises layers containing zinc alloys and pure zinc, there is no fear of galvanic corrosion when galvanized steel is in contact with other zinc coatings. Since zinc coating longevity is directly related to the coating thickness, the components with thinner coatings are likely to experience corrosion of the base steel first.
 [7]
[7]Preventing galvanic corrosion
Once the potential for and associated severity of galvanic corrosion is addressed, what are typical measures to avoid accelerated corrosion of any galvanized steel components? In theory, preventing galvanic corrosion is simple because it is achieved by interrupting only one component of the galvanic cell: the anode/cathode, the electrical connection, or the electrolyte exposure. In practice, the preferred solution will depend on project or design constraints.
Although it appears straightforward to specify the use of a single metal type within a project, often this is impractical because of availability or the material properties required for various building construction members. Where the direct combination of two different metals cannot be avoided, it is sometimes possible to select metals where the electrical potential between the anode and cathode are minimized. If evaluating the connection of aluminum-framed glass curtain walls to structural steel framing, hot-dip galvanizing the structural steel framing would reduce the electrical potential between the building frame and the glass curtain walls because aluminum and zinc surfaces are closer together on the galvanic series of metals than aluminum and carbon steel.
When reducing the electrical potential between the two metals in contact is unachievable, it is also possible to interrupt the electrical connection between them. This method involves placing electrically inert spacer materials with low moisture absorption between the metals such as neoprene, rubber, plastic, or nylon. Alternatively, the corrosion-inhibiting pastes, dried adhesives, and sealants can be used to separate the different metals (Figure 6). Spacer material selections should consider differences in design life and thermal expansion coefficients when compared to the base metals to avoid negative impacts on overall design life or intended performance. Specific to the specification of bolted structural connections, dielectric washers and bolt sleeves can be specified to achieve full isolation of the bolt, but all dielectric materials should be evaluated for compatibility with the loading condition (read “Opposites Attract: A Primer on Galvanic Corrosion of Dissimilar Metals,” by Christopher Hewitt, Alan Humphries, and Eric Twomey). For high strength connections, the impact on connection design and installation should be evaluated and quantified prior to the use of shims and washers if unaddressed by an existing standard (consult RCSC Research Council on Structural Connections, Specification for Structural Joints Using High-Strength Bolts, August 1, 2014).
As a third option, a galvanic cell can be interrupted by application of a durable liquid coating system to minimize exposure of the connected metals to the electrolytes in the environment. This method may be specified when electrical isolation is infeasible (slip critical connections, welded connections, etc.). Theoretically, painting only one of the exposed metals is enough to interrupt the galvanic cell. However practical measures often necessitate painting both metals to avoid accelerated corrosion concentrated in the areas of any coating imperfections on the anodic metal (such as the galvanized members). Although a viable method of preventing galvanic corrosion, regular maintenance of the paint is required to maintain barrier protection and minimize exposure of the more active metal.
Conclusion
Though galvanic corrosion has a reputation for being a very dangerous and complex corrosion phenomenon, the options for mitigation and prevention are straightforward: utilize compatible metals and/or favorable surface area ratios, provide barriers to break electrical contact between metals, or isolate the metals from the environment. It is best to not let the fear of galvanic corrosion prevent design professionals from achieving long-term corrosion protection and multifaceted metallic aesthetic using HDG steel. Understanding the causes of galvanic corrosion and when to implement the preventive measures will allow hot-dip galvanizing to be specified in an imaginative and sustainable way that can be preserved for generations to come.
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2020/08/Cliffwalk_Capilano_Suspension_Bridge-9.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2020/08/Galvanic_Series_2016_English.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2020/08/Bimetallic_Couple_2016_English.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2020/08/galvanic-scale-w-potential-energies-listed.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2020/08/HDG-and-Galvanic-Corrosion-Chart-2020.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2020/08/Cliffwalk_Capilano_Suspension_Bridge-4.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2020/08/Galvanized-to-Aluminum.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2020/02/Alana.jpg
- afossa@galvanizeit.org: mailto:afossa@galvanizeit.org
Source URL: https://www.constructionspecifier.com/avoiding-galvanic-corrosion-with-dissimilar-metals/
 [8]Alana Fossa is the senior corrosion engineer for the American Galvanizers Association (AGA). Fossa provides assistance to architects, engineers, fabricators, owners, and other specifiers regarding technical issues and the processing of hot-dip galvanized steel (HDG). She also manages AGA studies and research on performance, application, and processing of HDG steel. Fossa can be reached via e-mail at afossa@galvanizeit.org[9].
[8]Alana Fossa is the senior corrosion engineer for the American Galvanizers Association (AGA). Fossa provides assistance to architects, engineers, fabricators, owners, and other specifiers regarding technical issues and the processing of hot-dip galvanized steel (HDG). She also manages AGA studies and research on performance, application, and processing of HDG steel. Fossa can be reached via e-mail at afossa@galvanizeit.org[9].