Cleaning and maintaining stainless steel
by Katie Daniel | November 29, 2016 2:44 pm

by Catherine Houska, CSI
With appropriate specification, stainless steel can last the life of a building. However, as with any other material, unsightly surface deposits can accumulate after many years of service. Accidents, vandalism, use of inappropriate cleaning procedures, and installation issues can make surfaces unsightly, cause damage, or even lead to rapid surface corrosion. Surface restoration is often possible with the right remediation approach.
Since its invention over a century ago, stainless steel’s durable beauty has been repeatedly demonstrated. In the mid-1990s, more than 30 years of dirt, hydrocarbons, and other deposits had blackened the iconic upper floors of New York’s Chrysler (left) and Empire State buildings, but simple cleaning with products commonly found in household kitchens returned them to their original appearance (Figure 1).
The inherent corrosion resistance of stainless steel often makes it possible to restore surfaces after years of neglect when other materials may have suffered too much deterioration to make that feasible. This assumes an appropriately corrosion-resistant stainless steel and durable finish have been selected for the project, which have been the subject of this author’s previous articles. (Previous articles by this author for The Construction Specifier include “Proving its Long-term Mettle” [August 2016], “Avoiding De-icing Salt Corrosion” [January 2015], “Designing on the Waterfront” [November 2007], “Stainless Steel for Severe Coastal Environments” [September 2011], “Architectural Metal Corrosion: The De-icing Salt Threat” [December 2006], “Preventing Corrosion in Soil” [April 2006], and [co-authored with James Fritz], “Swimmingly Stainless Pool Design” [December 2005]. Visit www.constructionspecifier.com[1]. See also this author’s articles for the International Molybdenum Association (IMOA) e-newsletter, Stainless Solutions, along with Architectural Metal magazine.)
General guidance
Environmental factors influencing the frequency of the routine cleaning required to retain a pristine appearance include:
- the owner’s aesthetic standards;
- surface finish roughness;
- airborne particulate concentrations;
- pedestrian traffic levels; and
- exposure to regular heavy rain.

Photo courtesy ATI Allegheny Ludlum
In exterior environments, sheltered areas (e.g. balconies or the lower floors on high-rises) can face more aggressive environments because rainwater cannot wash off corrosive surface deposits. A more corrosion-resistant stainless steel, smoother finish, and increased maintenance may be necessary to retain an attractive appearance.
One should always request the cleaning product’s Globally Harmonized System of Classification and Labelling of Chemicals (GHS) information or material safety and data sheet (MSDS), and avoid chemicals containing ‘chlor’ (i.e. chlorides), acids, particulate, and anything potentially corrosive or abrasive. When there are concerns, a stainless steel supplier, industry association, or consultant can review the product chemistry before it is used. (A cleaning company, blogger, or random website does not necessarily understand metal corrosion or specialized finishes.)
Proprietary detergent and water solutions, including those used for automotive or dishwashing, and ‘environmentally friendly’ cleaning products containing hydrogen peroxide, vinegar, or similar chemicals are also used. The detergent should contain both a surfacent and degreaser, not leave a coating on the surface, and preferably be chloride-free and pH-neutral (i.e. non-acidic). Many cleaning products and wipes contain chloride compounds, such as bleach (sodium hypochlorite). If such products are used, the chloride or bleach content should be less than three percent, and thorough rinsing to remove the chlorides is critical. Bleach concentrations of five percent or higher cause corrosion of commonly used stainless steels like Type 304/304L at room temperature, so it is critical not to let solutions dry and concentrate.
Wash water
Clean, potable water is used for rinsing surfaces after most cleaning procedures, but it is important to check the water’s chemistry. The U.S. Environmental Protection Agency (EPA) suggests no more than 250 ppm for chlorides and 500 ppm for total dissolved solids (TDS) for human consumption, but there are no hard maximums. In some areas, these levels are much higher, which could add to both corrosion and hard water staining problems.
Suitable water may need to be purchased or a reverse-osmosis (RO) system installed. It is important never to use natural untreated, industrial, or swimming pool water. When acidic cleaning products are used, the rinse water should have a maximum TDS content of 200 ppm or be de-ionized, distilled, or RO water—otherwise, hard water staining occurs (Figure 2). While it can be removed, opting for avoidance is far more cost-effective.
Applying cleaning products
Even durable finishes can be damaged with inappropriate cleaning methods—this is a particular concern for fragile mirror and colored finishes. Too often, ‘cleaning’ is attempted with abrasives only appropriate for refinishing. One should use a new or clean, soft, lint-free cloth or a clean sponge reserved for exclusive use on stainless steel. It is critical to avoid cleaning products used on other materials, such as carbon steel.
Products that can potentially change the finish appearance, or contaminate the surface with iron, include:
- coarse abrasives pads (e.g. sandpaper or non-metallic abrasives);
- metal scrapers, brushes, or wool pads;
- coarse abrasive powders; and
- abrasive blast media. (See Figure 3 for an example.)
A soft nylon brush or plastic scraper can be used to help loosen adherent surface deposits, but should be tested first to ensure against surface damage. When cleaning directional finishes, one should always rub along the grain (Figure 4).
Light dirt, urine, and fingerprint removal
The choice of cleaning method for removing surface deposits, fingerprints, and other light discoloration depends on the application. Hand cleaning is common, but hot-water power-washing is appropriate for exterior applications where water infiltration is not a concern and a fast, low-cost cleaning option is desired.
Light fingerprints and dirt accumulations are also easily removed with common window cleaning products, such as ammonia and water or vinegar and water solutions. This makes it easy to clean adjoining stainless steel surfaces as windows are cleaned. These products are also suitable for other light cleaning requirements, but they will not remove heavier fingerprinting (Figure 5).
Mild detergent and degreaser solutions will increase cleaning effectiveness. If there are chlorides (coastal or de-icing salts) on the surface, cleaning effectiveness is increased by a proprietary additive specially formulated to improve removal.
Heavy fingerprints, grease, and oil
Heavy grease and oil deposits can be removed with vapor or steam de-greasing, high-pressure water jets, or alkaline or emulsion cleaners. Hot-water power-washing with a mild detergent or oil-free citric acid solution can also be effective. Some household oil-free citric acid cleaners and degreasers effectively remove many heavier fingerprint, oil, and lighter grease deposits.
Proprietary industrial strength degreasers, such as alkaline formulations with surfactant additions, are effective on heavier oil and grease deposits. Any new product should be tested on a small stainless steel surface before use to ensure it does not cause color change. Manufacturer instructions for application and surface rinsing must also be followed.
Clear coatings, oil, and wax
Stainless steel provides the best corrosion resistance when the surface is clean and exposed to oxygen. Clear coatings prevent oxygen exposure and can potentially cause corrosion problems and increase maintenance costs. The most problematic coatings are those that peel or delaminate; they create crevices as they fail, increasing corrosion problems.
Coatings increase surface reflectivity and can yellow over time. When applied in the field, service life is typically relatively short; repeated removal and replacement can be more expensive than simple cleaning. Further, some require such hazardous chemicals for removal that contractors frequently remove them by abrasion, destroying the initial surface.
If a coating must be applied to hide fingerprints or improve corrosion performance, one should select products that naturally dissipate or are easily removed to avoid finish damage—examples include oil, wax, and silicon mixtures. With the exception of lanolin polishes that dry hard and add natural corrosion protection, oils should not be used in exterior applications, swimming pool environments, or any other location with airborne dust or corrosive substances (e.g. salt or pollution), as they increase surface accumulations and can cause corrosion. Carnauba wax and similar automotive waxes that dry hard are also acceptable, but do not provide a corrosion-inhibitor.
Oil, wax, and silicon coatings can be helpful in indoor locations where fingerprinting is a concern. It is important to select products carefully since some do not harden and accumulate dirt (Figure 6).
Hydrochloric acid
Hydrochloric acid (i.e. muriatic acid) is very corrosive to construction materials; it should never be used for cleaning tile, concrete, or masonry near stainless steel. Concentrations of as little as 0.1 percent can cause room temperature corrosion of Type 304/304L (UNS S30400/S30403).
If there is accidental exposure, the surface should be immediately and thoroughly rinsed with clean water and the acid should be neutralized (Figure 7). Alternative cleaning products are available.
Adherent deposits
Degreasers can be very helpful in loosening some adherent deposits not involving adhesives. If the finish is not mirror-like, colored, or coated, then very fine abrasive powders suitable for cleaning glass can be effective when made into a paste and gently rubbed on the surface. (They should first be tested on a small area to make certain no surface damage occurs.)
The surface must be rinsed thoroughly to remove the white powder residue. A soft cloth or nylon brush can be used to loosen the powder. Calcium carbonate, which is used in toothpaste, is preferred because it does not scratch most finishes and is environmentally neutral. Fine crystalline silica, pumice powders, and baking soda (sodium bicarbonate) are also used. Coarse scouring powders should be avoided as they can contain bleach and can scratch surfaces.
Adhesive removal
Removal of residual adhesive deposits from protective strippable films, posters, and other sources can usually be accomplished without damaging the stainless steel surface. If the supplier can be identified, it should be contacted for removal advice. Several different adhesives are used in construction, and the appropriate removal products vary.
When recently applied, some can be removed with an eraser, mild detergent, vinegar (or ammonia), and water mixture. Plastic bristle brushes and scrapers may assist in removal, but anything that could scratch the surface should be avoided. Non-toxic household adhesive removers are also often very effective. If the finish is not mirror-polished or colored, fine abrasive cleaners suitable for glass can be made into a paste and then gently rubbed on with the grain to assist in removal. A strong solvent may be required, but it should be tested on a small area in advance and washed off completely afterward (Figure 8).
Sealant failure
‘Rundown’ occurs when fluids are released from sealant, producing dark areas or streaks below the joints as dirt, hydrocarbons, and other substances in the air collect on the tacky surface. The causes can range from poor installation to chemical exposure to normal end-of-service-life deterioration. (See the Failures article from the April 1997 issue of The Construction Specifier, “Premature Sealant Failure,” written by David H. Nicastro and Joseph P. Solinski.)
Discoloration color is determined by the type of particulate adhering to the sealant. This aesthetic problem is different in appearance from the normal rain/dirt runoff patterns occurring at window corners or directly under a joint (Figure 9). The appropriate removal product depends on the sealant type.
Paint and marker pens
Paint and marker pen stains can be removed using proprietary alkaline or solvent paint-strippers after testing the product on a stainless steel sample or in a low-visibility area to ensure it does not cause any surface discoloration. A soft, nylon bristle brush can be helpful in loosening residue. Some proprietary chemical cleaners can damage sealant—care should be used to prevent inadvertent damage.
Cement and mortar
If cement or mortar is accidentally spilled onto stainless steel, it should be washed off immediately with adequate water before it can set. Otherwise, removing solidified material can be difficult without causing surface damage. If the surface is smooth, it may fall off as it dries. Low-power-washing can also be tried, with the water stream angled to loosen the deposit edge.
If the cement or mortar has been allowed to dry on the surface, dark multi-color alkaline staining may be apparent on the stainless steel surface after the deposit is removed. This can be removed by rubbing a paste of fine abrasive powders and water on the surface. However, if the stainless steel surface is colored or coated with metal, permanent surface damage may occur (Figure 10).
Conclusion
Appropriate cleaning of stainless steel can frequently restore the original appearance of stainless steel. There is generally no reason to use products damaging to the environment or hazardous to workers. Cleaning frequency is determined by the owner’s expectations, site conditions, and appropriateness of the stainless steel and finish.
Generally, only occasional cleaning is required to remove surface deposits. However, care should be taken in applying coatings since some can adversely affect corrosion performance. With appropriate procedures and products, stainless steel can stand the test of time. (The author would like to acknowledge the support of the Nickel Institute and IMOA in the preparation of this article.)
Catherine Houska, CSI, is a senior development manager at TMR Consulting. She is a metallurgical engineering consultant with an MBA specializing in architectural metal specification, restoration, failure analysis, atmospheric corrosion, finishes, and market development. Houska has authored more than 190 publications (including several features for The Construction Specifier), and received CSI’s 2016 Technical Document Award. She can be reached via e-mail at chouska@tmr-inc.com[9].
- www.constructionspecifier.com: http://www.constructionspecifier.com
- [Image]: http://www.constructionspecifier.com/wp-content/uploads/2016/11/Stainless_figure3.jpg
- [Image]: http://www.constructionspecifier.com/wp-content/uploads/2016/11/Stainless_figure4.jpg
- [Image]: http://www.constructionspecifier.com/wp-content/uploads/2016/11/Stainless_figure5.jpg
- [Image]: http://www.constructionspecifier.com/wp-content/uploads/2016/11/Stainless_figure7.jpg
- [Image]: http://www.constructionspecifier.com/wp-content/uploads/2016/11/Stainless_figure8.jpg
- [Image]: http://www.constructionspecifier.com/wp-content/uploads/2016/11/Stainless_figure9.jpg
- [Image]: http://www.constructionspecifier.com/wp-content/uploads/2016/11/Stainless_figure10.jpg
- chouska@tmr-inc.com: mailto:chouska@tmr-inc.com
Source URL: https://www.constructionspecifier.com/cleaning-and-maintaining-stainless-steel/

 [2]
[2]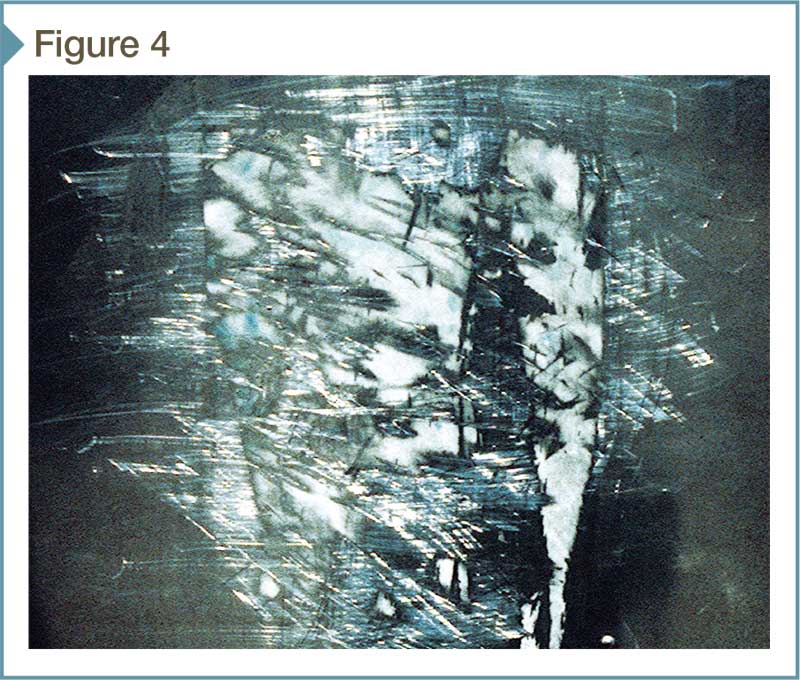 [3]
[3] [4]
[4]



 [5]
[5] [6]
[6] [7]
[7] [8]
[8]



