Corrosion resistance and environmental considerations for architectural metal coatings
by sadia_badhon | January 17, 2020 8:34 am
by Scott Moffatt
 [1]
[1]The coil and extrusion coatings industry is at a crossroads. Certification and testing bodies such as the American Architectural Manufacturers Association (AAMA) and ASTM have introduced stringent corrosion testing to better reflect real-world environments. Currently, there are discussions about raising industry standards for corrosion resistance in coastal and industrial environments. At the same time, architects, governments, municipalities, and also environmental organizations are demanding the elimination of materials on the Living Building Challenge’s (LBC’s) Red List, such as chromium and lead, traditionally used as corrosion inhibitors. This strategy, while well intentioned, has the potential to reduce corrosion performance of coated metal building components in hot, humid, salt-laden coastal areas and industrial environments.
The coil and extrusion coatings industry is committed to developing products offering increased corrosion performance and do not contain the Red List materials. While a comprehensive and cost-effective solution remains elusive, the industry is working to resolve these issues.
Corrosion and causes
The definition of corrosion is “the chemical or electrochemical reaction between a material, usually a metal, and its environment that produces a deterioration of the material and its properties.” Multiple factors can accelerate corrosion including:
- salt-saturated air and salt spray from oceans;
- high humidity;
- condensation (dew);
- sunlight;
- impact, freeze/thaw;
- time of wetness;
- industrialization (salt from de-icing roads, acid rain/smokestack emissions, and vehicle exhaust);
- dew, rain, and floods;
- birds and insects;
- fungus, bacteria, microbes, plant sap, and mildew;
- temperature and sunlight variation;
- dust, hail, lightning, gravel impact, abrasion, high-pressure spray;
- poor surface preparation;
- insufficient maintenance; and
- human perspiration.
Filiform corrosion
 [2]
[2]There are many forms of corrosion associated with the building market. One of the most prevalent is filiform corrosion. It mostly occurs on aluminum substrates. This type of corrosion begins when a substrate metal is exposed by a deep scratch, or when a cut edge enables moisture to penetrate beneath a metal coating.
Filiform corrosion requires relative humidity (RH) measuring between 40 and 90 percent. It spreads in streaks and creeps along the surface of the metal, causing the coating to lose adhesion and its aesthetic appeal. Filiform corrosion accelerates in warm temperatures, high humidity, elevated salt environments, and when metal is exposed by a raw or cut edge (Figure 1).
Aluminum extrusions and coil-coated materials are both susceptible to filiform corrosion because the metal edges are frequently exposed. Most aluminum extrusions are fabricated after coatings are applied, so when window frames are mitered, or fastener holes are drilled during installation, exposed edges may be left unprotected by the coating.
The vulnerability of exposed edges is also an issue for manufacturers applying paint to coil-coated stock. When paint is roll-coated onto the flat surface of the coil, edges are left uncoated. The same phenomenon occurs when painted coils are cut and fabricated into sheets. Fabrication bends in these applications may cause micro-cracking on the paint surface, opening another site for moisture penetration.
Galvanic corrosion
Galvanic corrosion occurs when electrochemically dissimilar metals share an electrically conductive path, enabling the ions from one of the metals to attack and oxidize the other. Applying a continuous paint film (coating) or inserting a gasket eliminating contact between two or more incompatible metals helps prevent this type of premature corrosion.
Film erosion
 [3]
[3]Film erosion is most commonly associated with coatings that are not ultraviolet (UV)-durable and, therefore, susceptible to chalking. Severe chalking causes paint film to deteriorate or erode at a high rate, ultimately exposing the metal substrate underneath the paint to moisture and other hazards that initiate the corrosion process.
Geography of corrosion
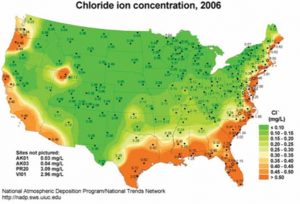
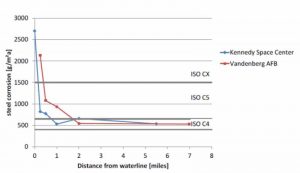
As Figure 2 illustrates, buildings and metal structures along the U.S. Gulf Coast and Eastern Seaboard are susceptible to corrosion failure due to the preponderance of heat, humidity, salt, and wind. According to most architectural metal coatings manufacturer warranties, a seacoast region is defined as an area from 457 m (1500 ft) up to 1.5 km (1 mi) inland from the coast. As the chart in Figure 2 indicates, salt concentration drops significantly after 1.5 km inland, as defined by the level of sodium and chloride ions in the atmosphere. However, moist, salt air from the sea can carry well beyond those distances. In fact, wind (at times, hurricanes) have been known to carry sea salt several hundred miles inland from coastal areas.
While that reality makes buildings throughout the state of Florida vulnerable to corrosion failure, the structures most susceptible to such damage are located in areas experiencing high levels of crashing surf and salt mist. Luckily, abundant rainfall in the Sunshine State and other southern coastal regions helps mitigate corrosion by rinsing salt residue from buildings (although any metal building parts located under an eave or shielded from rainfall remain vulnerable to corrosion).
Buildings in northeastern U.S. are less exposed to the corrosive warm and humid conditions than those in Florida and southeastern U.S., yet the proliferation of winter road salt can yield similar results. In large cities, such as New York, Boston, and Philadelphia, road salt has been shown to reach surfaces as high as 20 stories from the ground, due to the wind tunnel effect created between tall buildings.
 [4]
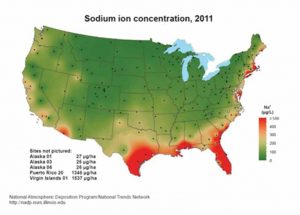
[4]While all coastal locations are susceptible to salt air corrosion, the West Coast is not as severely affected due to the dryness
of the air. Filiform corrosion requires humidity in excess of
40 percent to develop. Nevertheless, corrosion maps show salt can travel from the Sea of Cortez in the Baja Peninsula to noncoastal areas as far inland as southern Arizona.
Current industry standards
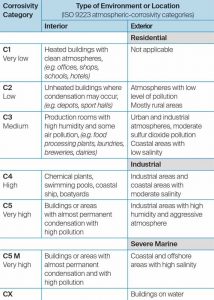
The International Standards Organization (ISO) has developed standards to rate interior and exterior environments according to their respective degrees of risk for corrosion (Figure 3). Ratings within ISO 9223, Corrosion of metals and alloys – Corrosivity of atmospheres – Classification, determination and estimation, are determined by weighing a steel panel, exposing it to a specific environment for one year, and then re-weighing the panel again to measure the degree of weight loss that occurred during that time.
For example, an exterior environment designated as C2 (C stands for corrosion) represents a rural area with low levels of pollution, while an exterior environment with a C5-M designation identifies a harsh coastal environment with high levels of salt air.
Accelerated corrosion testing and AAMA standards
AAMA has also developed industry standards for various levels of corrosion performance, commonly referred to as AAMA 2603, Voluntary Specification, Performance Requirements and Test Procedures for Pigmented Organic Coatings on Aluminum Extrusions and Panels (with Coil Coating Appendix), AAMA 2604, Voluntary Specification, Performance Requirements and Test Procedures for High Performance Organic Coatings on Aluminum Extrusions and Panels (with Coil Coating Appendix), and AAMA 2605, Voluntary Specification, Performance Requirements and Test Procedures for Superior Performing Organic Coatings on Aluminum Extrusions and Panels (with Coil Coating Appendix). Each standard contains laboratory-based accelerated test methods that were adopted to mimic, as closely as possible, corrosive conditions in the real world.
Historically, the primary test method had been the Salt Spray Fog Test per ASTM B117, Standard Practice for Operating Salt Spray (Fog) Apparatus. During this test, coated panels are exposed in a chamber to a constant mist cloud containing a five percent sodium-chloride solution at a sustained temperature of 35 C (95 F). The test requirement to meet AAMA 2605, the industry’s most rigorous performance standard for weathering performance, was 4000 hours. Recently, AAMA eliminated the 4000-hour salt spray specification, determining a new accelerated corrosion test method—the G85 Annex 5 cyclic corrosion test—better mimics real-world corrosion.
Corrosion-resistant pretreatment technologies
For the past 50 years, the building industry has relied on zinc-coated steel and/or chromium pretreatments in combination with chromium primers to protect steel and aluminum building components, especially from corrosion, in industrial and coastal environments. Chrome also enables coatings to adhere more strongly to the metal surface.
 [5]
[5]Chrome pretreatment, combined with a strong-adhering chrome primer, is one of the most effective coating solutions for preventing the proliferation of filiform corrosion on aluminum substrates. Although chrome pretreatments and primers will not stop filiform corrosion from occurring, they do prevent it from proliferating.
Three pretreatment options most commonly used for aluminum substrates are:
- hexavalent chrome;
- trivalent chrome; and
- chrome alternatives.
Hexavalent chrome
Hexavalent chromium is also referred to as “hexavalent chrome Cr(VI),” “Cr6” or “chrome-six.” It has a gold, yellow, or green appearance and offers the most robust corrosion protection resistance. It has been used in the industry for 50 years.
While hexavalent chrome is one of the most robust options for corrosion protection, it also is the least safe for the environment and human health, as it is both toxic and carcinogenic. Due to its toxicity, hexavalent chromium has been placed on the Red List of products to avoid by administrators of LBC, one of the world’s most rigorous green building certification systems. Other environmental organizations often reference the Red List when creating their own restricted substance lists.
A major performance benefit associated with this material is its wide operating window. AAMA specifications require minimum coating weights of 40 mg/sf for this type of pretreatment system, although many applicators apply up to 100 mg/sf to optimize protection. It is essential for applicators to find the coating weights best suiting the performance demands of their product or application. If the pretreatment is applied too thinly, it is vulnerable to corrosion, but if applied heavily, it can turn to powder and cause a loss of adhesion between the pretreatment and the paint surface.
Trivalent chrome
Trivalent chrome is referred to as “Cr3” or “chrome-three,” and features a lower concentration of chrome than hexavalent chrome. Trivalent chrome has proven to be an effective corrosion prevention solution, but it still does not facilitate the goal of fully eliminating heavy metals from the waste stream. Although it is not currently considered carcinogenic, trivalent chrome is still a heavy metal and likely to be placed on the Red List in the future.
Its appearance is clear, though additives are often used to indicate the presence of a coating. Trivalent chrome does not have the same level of corrosion protection or self-healing properties as hexavalent chrome, but it can be an effective corrosion deterrent if applied within the correct parameters.
Chrome, not intentionally added (NIA)
Pretreatments specifically formulated without intentionally added ‘chrome three’ or ‘chrome six’ have been developed and commercialized in recent years. While they offer excellent corrosion resistance, they are not as robust as legacy chrome alternatives.
In the applicator’s process, chrome (NIA)-containing pretreatment products have tighter operating tolerances. When these materials are run, they demand shorter test intervals to assure the chemicals are being applied at optimum performance levels. Operating outside of these rigorous tolerances can diminish the corrosion protection performance of the finished product. Failure to maintain tight operating conditions can lead to process failures and negative results. For this reason, it is important to test these pretreatment systems at frequent intervals during the application process to make sure the chemicals remain within specification. With chrome (NIA) they have to check, in some cases, every hour. Intervals vary by the pretreatment chemical company.
One proof of this phenomenon was evidenced years ago in the coil industry. Responding to demand for more flexible pretreatment options to help fabricators improve their post-forming operations, the coil industry began converting to complex oxide pretreatment systems. Before long, corrosion failures on metal roofing resulting from acid rain fallout became a problem in the ‘rust belt’ areas of the United States.
Continued rise of chrome pretreatment alternatives
With environmental regulations becoming stricter around the globe, there is a growing demand to fully eliminate not only chrome pretreatment, but also chrome primers and chrome-containing multilayer coatings systems from industrial and architectural coatings systems. This is especially true in the European Union (EU), where legislators have mandated the elimination of several hexavalent chrome compounds from most paint system by end of this year.
Most U.S. pretreatment systems use chrome phosphate, which has excellent adhesion but less throw (i.e. protection beyond application area) protection on the edges of a metal surface. Chrome-chromate, which is not often used in the U.S., has less adhesion strength but more throw power and can be considered self-healing.
In Europe, manufacturers are pursuing two different pretreatment alternatives. The first is improved etching with acid cleaners to remove more oxide from aluminum surfaces. While cleaning a metal surface is probably the most important step in preparing it for painting, removing aluminum impurities—along with oxides and oils—may be the most significant step in coating it. Acid-etching has proven to enhance the ability of coatings to adhere to aluminum surfaces, and thereby, improve their corrosion protection.
 [6]
[6]The second alternative is flash anodizing to impart a fine layer of protection to the surface of the metal to prevent the formation of filiform corrosion. Filiform corrosion cannot form on an anodized layer. Anodizing does not perform as well as coatings, but it can be good as a preparation for metal under the coating. Coatings adhere well to anodize before they are sealed. Adhesion is difficult after sealing. Also, flash anodizing does not seal.
Corrosion-resistant coatings systems
The need to protect metal from corrosion is not restricted to the substrate and pretreatment layer. This section summarizes the advantages and disadvantages of the following four major corrosion protection layers and/or systems and components for building products:
- pretreatments;
- primers;
- clearcoats; and
- one-coat systems.
Pretreatment
Real-world results and south Florida accelerated exposure testing have demonstrated coatings systems containing a layer of chrome in the pretreatment or primer will perform to expectations. Recent experience has shown non-chrome-containing systems can lead to serious corrosion and safety issues on buildings when improperly applied.
Primers
Primers are as important as pretreatment methods in protecting metal substrates from corrosion because they make coatings adhere more readily to metal surfaces and provide an excellent barrier against moisture penetration. While primers are formulated to adhere to metal and enhance corrosion protection, topcoats are engineered to emphasize UV protection, thus limiting their ability to achieve optimum adhesion.
Clearcoats
Clearcoats aid directly in the prevention of corrosion by functioning as an extra protective barrier against salt penetration. Additionally, clearcoats tend to rinse and clean more easily than color coats, which, due to the presence of pigmentation in the film, feature a rough surface that can trap and hold dirt and salt residue. Since clearcoats have smooth surface profiles, rain is effective in removing external contaminants from their surfaces.
Clearcoats are mandatory over metallic flake-containing paint systems because they prevent the aluminum flake from tarnishing and discoloring, but they can also be layered over solid and mica coatings to enhance UV protection.
Panels finished with clearcoats placed in south Florida testing farms have demonstrated virtually no change in chalking or color fade after 35 years of continuous weather exposure. Clearcoats eliminate chalking because they do not contain pigments. Pigments lose their chemical bond with coatings film during exposure to UV light. This is the primary condition leading to the formation of chalk.
One-coat systems
Single-layer powder and liquid coatings are becoming popular for less-rigorous AAMA 2604 architectural and building-product applications, where they frequently displace two-coat, 50-percent polyvinylidene fluoride (PVDF) coatings that have dominated this performance category for more than
50 years.
UV-durable fluoropolymer coatings are less susceptible to film erosion than standard polyester- and acrylic-based ones because they have powerful molecular bonds limiting film loss to less than 10 percent every 20 years. One-coat liquid and powder coatings for aluminum extrusions began entering the building market in the early 2000s to lower cost and meet environmental mandates.
While most applications were limited at first to residential and light building projects, architects eventually began to specify them for monumental buildings requiring compliance with AAMA 2604 and 2605 standards for weathering and corrosion resistance.
One-coat liquid products are also marketed for aluminum applications in the coil industry. Typically applied as clearcoats or tinted clearcoats over natural or brushed aluminum surfaces, one-coat liquid finishes accentuate the metallic appearance of aluminum and provide an anodized look to the coated part.
While one-coat liquid and powder systems are viable for normal building environments, they can be susceptible to premature corrosion in coastal environments when not applied over a robust base primer or if they are not manufactured under the proper operating conditions.
Other methods to reduce corrosion
Even in Florida, where rainfall contributes substantially to removing salt residue from building surfaces, fresh-water flushing is recommended for eaves, overhangs, and other metal building components not regularly exposed to weather. Countless cases of pitting and corrosion on metal building surfaces have been documented, with many failures traced to the release of sulfur gas from sealants and gaskets that have not been rinsed regularly. For this reason, coating manufacturer warranties typically recommend freshwater rinsing on a regular basis to keep such areas free from salt build-up. (For additional recommendations on keeping buildings free of corrosive materials, reference AAMA 609 and 610-15, Cleaning and Maintenance Guide for Architecturally Finished Aluminum.) Recommendations vary by location—once a year to as many as six times a year based on the salt concentration.
Conclusion
Legacy coating systems have demonstrated their ability to last more than 50 years on landmark buildings, and in harsh industrial and seacoast environments, since their introduction in the 1960s. Despite their proven effectiveness, there is a growing demand in the United States to eliminate harmful ingredients such as hexavalent chrome, trivalent chrome, and chrome primers from legacy coatings systems. Abolishing some or all these materials may have an adverse effect on long-term corrosion resistance and raise overall cost.
In the future, it will be important for pretreatment chemical and coatings companies to innovate and improve corrosion resistance with new alternative materials. Over the months and years ahead, it is likely new technologies will emerge, setting new standards of performance.
Scott Moffatt is market manager, building products for the coil, extrusion, and wood groups within PPG Industries. He has 40 years of experience within PPG’s industrial organization in various sales, marketing, and management functions covering multiple technologies and sectors. Moffatt’s current position involves getting PPG specified with architectural firms, glazing companies, and applicators. He can be reached at moffatt@ppg.com[7].
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2020/01/Morphosis-BloombergEx-5.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2020/01/Figure-1-Metal.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2020/01/Chloride-Ion-Concentration.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2020/01/Corrosion-Coastline-Chart.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2020/01/Salt-Concentration.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2020/01/ISO-9223-CHART.jpg
- moffatt@ppg.com: mailto:moffatt@ppg.com
Source URL: https://www.constructionspecifier.com/corrosion-resistance-and-environmental-considerations-for-architectural-metal-coatings/