| TESTING CONCRETE ALKALINITY |
| It is useful to keep in mind the moisture condition of the concrete slab is closely tied to pH—a measure of hydrogen ion concentration. This can be assessed quite simply using a pH meter.
When moisture is not an issue, pH should not be one either. Still, given highly alkaline conditions will typically lead to de-bonding and other serious flooring issues, it is generally good practice to test the pH, and many flooring manufacturers require it. |
Why using the moisture vapor emission rate test can be risky
The ASTM F1869 test does not give reliably accurate results because it is based on a faulty premise: namely that testing moisture at the concrete slab surface—where the CaCl2 crystals are placed—reflects the overall slab’s true moisture condition.
Several factors make surface moisture test results misleading. For example, ambient conditions can interfere with test results. Warmer or more humid room air can lead to higher test numbers, while colder or less humid air in the room can lead to lower test numbers. In either case, the test result may not reflect the actual moisture condition within the slab.
Further, the test measures the moisture level only at the top 12 to 18 mm (1⁄2 to 3⁄4 in.) of the concrete slab. Since moisture leaves through the top of the concrete, this uppermost layer is typically drier than conditions deeper within the slab.
Conversely, CaCl2 crystals can also attract a disproportionate amount of moisture from the concrete, making it seem like the slab has more moisture than it really does. This false result can then unnecessarily delay the floor installation.
Results only indicate the moisture condition at the top of the concrete slab at the time of the test, not the slab’s eventual point of moisture equilibrium. It is the moisture associated with this equilibrium that is the most useful indicator of whether the installed flooring will remain functional or buckle, crack, or suffer from other serious defects.
Engineers and inspectors in the United States first used the CaCl2 test—called the ‘dampness test’—as early as 1941. It involved placing a dish of CaCl2 crystals under a sealed glass dome and returning the next day to examine for dampness. This ‘eyeball’ qualitative assessment was employed for years without any studies to support its use, or the size and shape of the dish and dome. It was strictly a qualitative ‘go or no-go’ test without any associated numerical result.
Over the years, attempts have been made to quantify the test. Today, one measures the crystals and expresses the test result in terms of pounds of moisture per 1000 square feet for 24 hours, termed the moisture vapor emission rate (MVER). However, nothing in the scientific literature specifically points to a reliably safe threshold for application of adhesives or floorcoverings, whether 2 or 3 lb per 1000 sf in 24 hours, or some other threshold.
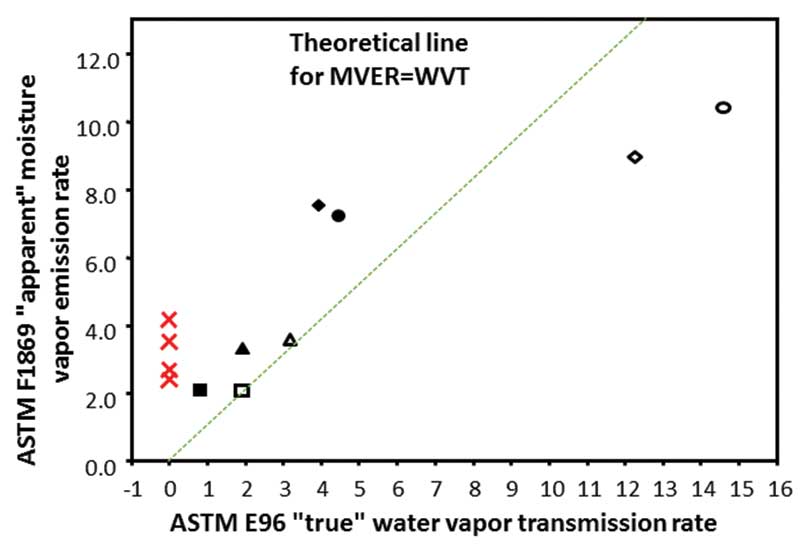
Image courtesy CTLGroup
Studying the MVER or CaCl2 test
To test the CaCl2 method more thoroughly, CTLGroup (a research body and subsidiary of the Portland Cement Association [PCA]) carried out a long-term study mimicking the test conditions.
Figure 2 shows some of the study’s results, comparing outcomes using the CaCl2 test to actual MVER. The diagonal line is a 1:1 ratio representing the line of accuracy for the test. Most points fall considerably far off the line, demonstrating the test is not particularly accurate. There is a tendency toward high test numbers at lower moisture levels, and low test numbers at higher moisture levels.
In addition, the four red Xs on the graph represent four concrete slabs that actually sat in the room at 50 percent humidity for several years. When they were weighed day after day, after several years, they were neither emitting moisture nor gaining moisture, and had a very low moisture level. However, when measured according to ASTM F1869, the results ranged from 2.5 to more than 4 lb. The desiccant in the CaCl2 kit was actually sucking out more moisture than was coming out of the concrete, giving a false positive result.




