Spotting the Differences: Five factors for variation in an anodize finish
by Katie Daniel | April 10, 2017 10:12 am
 [1]
[1]by Tammy Schroeder
Aluminum offers a wide range of properties and characteristics engineered to the demands of specific applications through choices of alloy, temper, and fabrication processes. When aluminum is anodized, it results in an extremely hard and durable surface finish. In fact, the anodized layer is second only to diamond in hardness. Along with its metallic appearance, 100 percent recyclability, excellent corrosion resistance, and minimal maintenance requirements, this makes it an excellent option for architectural building products PLACED in high-traffic areas such as storefronts, doors and entranceways, curtain walls abutting offices, and handrails.
Anodize coatings are created through an electrochemical process where the aluminum on the surface of a part is converted to aluminum oxide. The coating becomes integral to the part on which it forms. Therefore, the aluminum to be anodized has a significant impact on the finish. The aluminum alloy and temper, as well as the anodizing process, have profound effects on the success of achieving a superior, sustainable, visually appealing anodize finish.
Aluminum alloys and their alloying elements
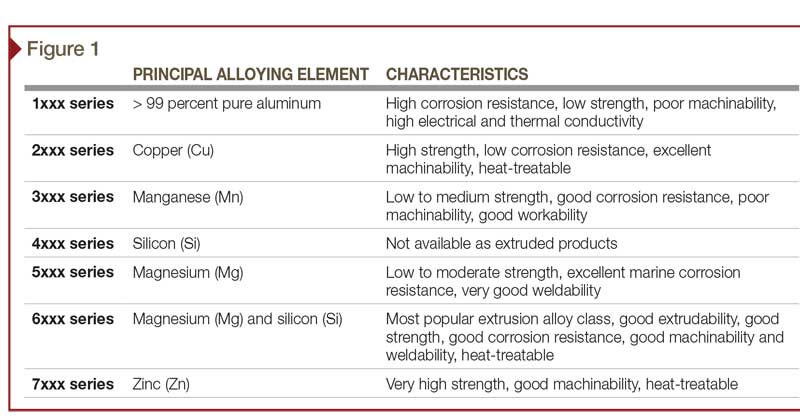
Pure aluminum is soft, light, corrosion-resistant, and highly conductive for electricity. Other constituents—such as silicon, copper, magnesium, manganese, chromium, and zinc—are added to aluminum to further enrich its properties (Figure 1). Predominantly, these additives can increase the material’s strength, workability, and response to finishing. Iron is also typically present as a contaminant, but it is desirable in very small quantities (as discussed later in this article).
Aluminum alloys, with their alloy constituents, can have a large effect on anodizing. The only component of an aluminum alloy that anodizes is the aluminum—all other components, elements, and impurities do not.
The 6xxx series are the alloys most commonly recommended and used in architectural and structural applications. They contain small amounts of magnesium and silicon as the principal alloying elements. The most frequently specified alloys, 6061 and 6063, contain no less than 95.8 percent aluminum. The total amount of other elements is less than five percent of the alloy composition. Impurities, such as iron, are also present, but their total percentage is usually less than 0.35 percent.
Anodizing is very dependent on the alloying elements. A slight variance in the aluminum’s composition can have profound impact on the finished appearance. Various combinations of constituent elements cause each aluminum alloy to react differently to the process of anodizing, through either surface chemistry or electrical conductivity. As a result, each alloy or series yields a different appearance, even if treated with an identical anodizing process.
 [2]
[2] [3]
[3]Photos courtesy Linetec
Mixing aluminum alloys means mixed results
An aluminum alloy is a chemical composition where other chemical elements are added to pure aluminum to enhance its properties and increase its strength. The finished extrusion’s characteristics are partly determined by the alloy’s composition. Each designation has a predetermined mixture of one or more elements together with the aluminum; they are typically added in amounts ranging from 0.05 to seven percent.
The alloy composition and the method of production have a substantial effect on the aluminum’s performance and its post-anodized appearance. The production method has an impact on the alloy’s final temper, which is attained through numerous forms of mechanical and thermal treatments such as the extrusion process, heat-treating, and quenching (i.e. manipulating properties through heating the metal and controlling the rate of cooling).
Whenever possible, extrusions from the same lot and temper should be used. There can be slight variances in the alloying elements and impurities between lots, which can affect the anodized finish. If it is not possible to use the same lot throughout the entire project, one should ensure the same lot of material is used per elevation. This is particularly important when working with sheet material such as 5005 alloy.
Aluminum temper
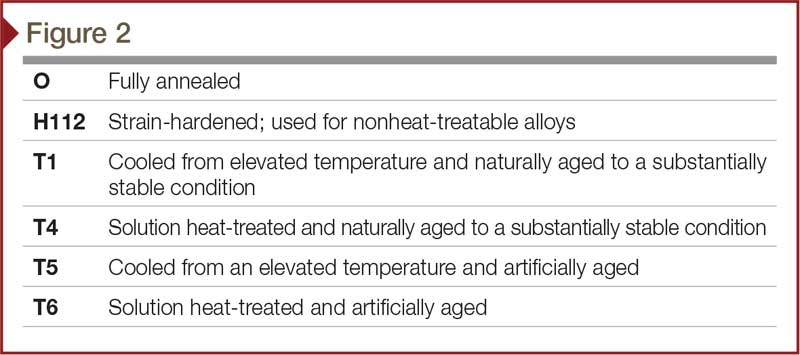
The word ‘temper’ refers to the combination of hardness and strength imparted to aluminum by mechanical or thermal treatments (Figure 2). Aluminum alloys are offered in a wide range of tempers. Those classified as ‘heat-treatable’ gain strength through the addition of heat. On the other hand, alloys classified as ‘nonheat-treatable’ develop maximum strength characteristics through cold-work hardening after extruding. This involves shaping the work piece at a temperature below its recrystallization temperature, usually at ambient temperature. In most cases, such cold-forming is done at room temperature.
Heat-treatable alloys, including the 6xxx series, attain their maximum strength through controlled heat treatment. They have the highest strength of aluminum alloys. The temper level of an aluminum extrusion has an impact on the anodizing process outcome because it affects the gloss and clarity. Typically, the higher the temper is, the higher the finish clarity will be. Temper also has an effect on the alloy’s surface chemistry, which can have an effect on the anodize color. To minimize variance in the anodize process, a project’s extrusions should be the same temper.
 [4]
[4]Primary versus secondary aluminum
Aluminum recovered from scrap (i.e. secondary aluminum) has been an important contribution to the metal supply since the 1950s. Economic implications and increased concern regarding the energy supply have focused even more attention on recycling of aluminum because of its energy-intensive nature. However, recycled aluminum can have higher concentrations of impurity elements, contaminants, and other metal constituents such as iron.
Iron is the most common impurity found in aluminum. Higher concentration of iron in the aluminum can cause excessive die wear and surface defects in the anodizing. However, too low of an iron concentration can cause a higher gloss on the anodized surface. Testing has shown an iron content of 0.15 to 0.2 percent provides the best response to the etch process. Secondary billet tends to have a higher concentration of iron.
An anodize finish on pure aluminum would be continuous and transparent, evenly transmitting light through the thin oxide coating. The contaminants and non-aluminum metals within the alloy of the aluminum extrusion can give clear anodize a darker finish. These areas contain particles of constituents and oxidation products detracting from the coating’s transparency. This indicates how important—or rather, detrimental—impurities are to the result.
The greatest complexity with secondary aluminum is the inconsistency in the alloy, as each lot can be significantly different. The impurities of secondary billet are higher, but still must be within the contaminant and element specification tolerance.
 [5]
[5]Chemistry of the anodize process
To produce a clear anodize finish, aluminum parts must go through a minimum of 12 process tanks. If colored anodize is needed, two additional tanks are required. Temperature, bath time, and chemical concentration of each tank must be tightly controlled in order to maintain a consistent process and finish. Automation of the anodizing process (and of each tank’s chemistry) is the best way to perform this.
The pre-anodizing steps of cleaning, etching, and deoxidation (or desmut) are intended to yield as much oxide-free aluminum as possible, as well as result in a uniformly conductive distribution at the surface for anodizing. Rinsing is imperative between process steps to keep contaminants out of subsequent process tanks.
Focus on the etch
The etching temperature, time, chemical concentration, and dissolved aluminum concentration are the most important etching process parameters influencing surface uniformity. The etching process’ goal is to produce an appearance that is homogeneously matte and consistent.
During anodizing, an electrochemical reaction converts aluminum into a porous aluminum oxide film. This transparent-to-translucent layer is several times thicker than the naturally occurring oxide. Light is scattered, refracted, and reflected differently from this film depending on the compositional variations, incorporated particles, and microscopic roughness of the aluminum’s substrate surface. However, during the alkaline etching process prior to anodizing, further surface roughness is also generated due to uneven chemical attack on the microstructure. The additional surface roughness created on the extrusion surfaces is due to different reaction rates between the alloy’s impurities or constituents and the aluminum substrate.
It is critical to control the etch bath parameters to decrease the intensity of, or even prevent, the occurrence of surface streaks. The final appearance usually depends on a good etch. An acid etch bath with fluorides produces a more matte finish—hence a more uniform finish—than a caustic etch.
Anodizers have varying control over their tank chemistry, tank temperatures, solution concentrations, and the time material spends in each tank. However, they have no control over the alloy, temper, or shape of the aluminum parts. These variables can make it extremely difficult to achieve an exact color from run to run and load to load. Working with a company whose processes are automated, with tight process controls, can greatly reduce these variables’ impacts.
 [6]
[6]Surface preparations
Defects to the surface of an anodized substrate can be introduced by various unrelated processes such as machining, sanding, or welding, or by residues from previous processes. Shot-peening, grit-blasting, or mechanical finishing techniques may leave residue on the substrate that can also introduce interfacial contamination and obstruct anodic oxide formation during the anodizing process. Laps, seams, and burrs created on the surface by these processes have distinct charge distribution effects on the substrate.
Anodizing can have an unpleasant effect on welds. When two or more parts are welded together, acid can be entrapped in the weld and in the area around the weld. Further, the heat developed from the welding process changes the metallurgy of nearby metal or heat-affected zones (HAZs), causing localized discoloration after anodizing—this is often referred to as a ‘halo’ or ‘ghost’ effect. The area around the weldment can be slightly lighter or darker in color, causing the welded area to appear larger than it is.
The most extreme finish variations exist when a welding rod alloy vastly differs from the alloy used to make the part. The proper 5356 alloy welding wire and the lowest heat possible should be employed when welding 6xxx series alloys. The worst choice is 4043 because it turns smutty black when anodized.
Some extrusion surface defects, such as pick-up and die lines, may not be completely removed from the etched surface, and their remnants become a part of the surface imperfections. For best anodizing results, there are a few other considerations to keep in mind.
 [7]
[7]Perform as much bending and forming as possible prior to finishing
As anodic films are very hard, most post-production forming or bending causes the film to ‘craze,’ which produces a series of small cracks in the finish, giving it a spider-web-like appearance.
Prevent solution entrapment
Proper drainage holes are essential for drainage of solutions, allowing entrapped gas to escape from the parts. Even the tightest welded joints cause anodize chemicals to weep out, causing undesirable defects such as streaks and potential coating degradation at the site.
Talk racking
The finisher needs to know where parts can be racked. Contact marks will be visible on the aluminum. The design professional must define what is acceptable and what is not with regard to exposed surfaces and rack marks. It is important to keep in mind the parts cannot simply be hung on hooks.
Store aluminum material properly
Aluminum should be stored in a dry, controlled environment. Moisture should never build up between the pieces—this causes severe corrosion that will not be removed in the finishing process.
Avoid adhesives
Tape or adhesive on the aluminum may leave a residue that may not be removed in the anodize process.
 [8]
[8]Photo courtesy EXTECH
Work with the right anodizer
An anodizer who utilizes fully automated anodize lines to reduce inconsistencies in the process can be an ideal partner. However, it is critical to keep in mind samples provided from an anodizer may not be the same alloy and temper as the aluminum used on the project—essentially, they may not be an exact representation of the finish achieved.
Always wear gloves
One should never handle unfinished aluminum with bare hands—oils on skin can cause permanent and severe corrosion.
Conclusion
For architectural applications, surface appearance is one of the most important characteristics of high-quality aluminum extrusions. By following this article’s guidelines for anodized aluminum, specifiers can help ensure the result is a visually appealing, sustainable, extremely hard, and durable surface finish contributing to a building’s lasting, positive impression and low-maintenance life cycle.
Tammy Schroeder, LEED Green Associate, is a senior marketing specialist at Linetec and serves as an industry educator on architectural coatings. Possessing 18 years of experience in the finishing industry, she shares her knowledge of liquid paint coatings, powder coat, and anodize with architects, specifiers, and architectural product manufacturers in commercial and residential markets. Schroeder can be reached via e-mail at tammy.schroeder@linetec.com[9].
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2017/03/anodized_Voxman_UniversityOfIowa13-e1490982671144.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2017/03/CS_Apr_2017_HR-63.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2017/03/anodize_Linetec_AnodizeLine.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2017/03/CS_Apr_2017_HR-64.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2017/03/anodize_Linetec_AnodizeRing2.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2017/03/anodize_AluminumBillet.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2017/03/anodize_Linetec_ClearAnodizeMaterial2.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2017/03/anodize_Linetec-EXTECH_MA_Logan1.jpg
- tammy.schroeder@linetec.com: mailto:tammy.schroeder@linetec.com
Source URL: https://www.constructionspecifier.com/spotting-the-differences-five-factors-for-variation-in-an-anodize-finish/