Why Red Brick Turns White: Understanding efflorescence
by Catherine Howlett | November 1, 2012 12:24 pm
 [1]
[1]Efflorescence is one of the first signs of moisture problems for cementitious materials, especially masonry. A by-product of moisture combining with free salts, this phenomenon is not only just a cosmetic problem—left unchecked during freeze-thaw conditions, it can cause brick to weaken, spall, or crumble in some cases.
 [2]
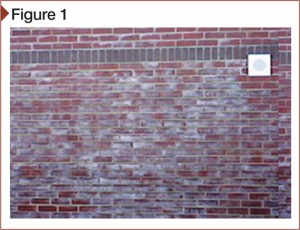
[2]Depending on its source, efflorescence is usually a white powdery substance (Figure 1). It forms on different cementitious and clay construction when internal, ‘restless’ moisture dissolves free water-soluble salt deposits in the masonry and carries them to the surface (Figure 2). The moisture and salts can also promote galvanic action. When the moisture containing the dissolved salts evaporates, efflorescence appears.
The type of salt compound in the structure dictates the appearance—alkali salt compounds usually found in mortar and cement products (e.g. sodium or potassium) produce the more common whitish to light gray deposits, but there is a wide range of variation. Depending on the type of salt compound in the structure, it can also range from shades of brown and green; it can be powdery, crystalline, dull, hazy, or bright.
According to the Brick Institute of America (BIA) and at least one masonry cleaning/sealing product manufacturer, metallic salt compounds from vanadium, manganese chromium, and molybdenum tend to produce discoloration (i.e. stains) other than white or gray. These compounds are often used as a colorant in specialty brick.
Some discolorations are often referred to as ‘stains’ because they usually occur during chemical cleaning, are more difficult to remove, and can be permanent. For instance, vanadium is greenish, while manganese tends to be brown streaks resembling tobacco stains (Figure 3).
Lime run is another discoloration very similar to, and often confused with, efflorescence. It usually forms in the joints and runs down the face of the masonry units during cleaning (Figure 4). The salts and resulting colors are different, but the production processes are basically the same.
Efflorescence 101
Efflorescence can cover large surface areas, individual masonry units, or partial brick. These reactions bring the salts to the surface in a solution that forms crystals when it combines with carbon dioxide (CO2) in the air.
 [3]
[3](B) Efflorescence caused by improper drainage and no waterproofing behind the retaining wall.
(C) An improper metal canopy tie-in directed moisture to the concrete lintel, causing efflorescence, spalling, and deterioration of attempted repairs.
(D) Efflorescence on shaded clay pavers over an unprotected base.
(E) Efflorescence mixed with traces of mildew on shaded concrete pavers caused by moisture migrating from the unprotected base.
(F) Moisture mixed with the salts in a clay pot planter that was kept in the shade and formed a heavy crust of efflorescence.
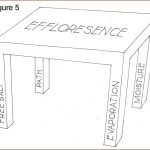
The term ‘efflorescence’ is not always properly pronounced (“eff-floor-ess-sense”), much less adequately understood by design/construction professionals. It is the result of the combination of four factors:
- salt deposits;
- moisture;
- moisture path to the surface; and
- evaporation.
As shown in Figure 5, it is analogous to a four-legged table where eliminating any one of the legs causes collapse. Remove one ‘leg’ and efflorescence is stopped.
Hidden efflorescence can be present in masonry when the salts are dissolved by moisture, but the moisture has not migrated to the surface. A rudimentary form of efflorescence can be made by sprinkling some table salt (i.e. sodium chloride) in a clear glass and adding water—the salt then dissolves. When the water is poured out, and the residual moisture in the glass evaporates, a cloudy white salt film is left on the glass (Figure 6).
The powdery form of efflorescence can usually be brushed away. However, when it is allowed to go through repeated cycles of being deposited, rain-dissolved, and re-dried, it becomes crystalline, tightly bonding to the surface.
Efflorescence is unsightly and is usually a source of disagreement between architects, designers, builders, and owners as to why it occurs and what should be done when it appears. Indeed, according to ASTM C1364, Standard Specification for Architectural Cast Stone, efflorescence alone on manufactured units is not a reason for rejection. Still, if allowed to progress, the result can be spalling that affects masonry’s structural and durability properties.
It is not always possible to predict whether efflorescence will occur. Soluble salt components may be present in concrete, mortar, brick, concrete masonry unit (CMU), cast stone, portland cement stucco, or earth. They may be carried into the wall with any form of precipitation, fog, condensation, sprinkler systems, or be absorbed by groundwater.
 [4]
[4]When this author tested the pH of masonry construction, areas without efflorescence had a pH ranging from 8 to 12; areas exhibiting efflorescence were above 12. Most paint manufacturers recommend cementitious materials have a pH of less than 12 before painting. They also recommend using an alkali-resistant primer when painting cementitious products.
Most efflorescence is temporary and consequently should be left alone. It often occurs shortly after building wash-down, and in the fall and winter when vapor transmission slows down and masonry stays damp for extended periods. Since most masonry cleaners tend to be acidic, acid rain is often a natural remover of efflorescence.
Evolutionary lesson
Beginning with calcium chloride that usually comes from lime, the following four salt compounds evolve to form efflorescence:
- calcium oxide (soluble in water);
- calcium hydroxide (soluble in water);
- calcium carbonate (insoluble in water); and
- calcium bicarbonate (soluble in water).
When calcium oxide is mixed with water, it forms calcium hydroxide; it is then carried to the surface
by normal moisture migration, mixing with carbon dioxide in the atmosphere to form calcium carbonate. This is the first visible white indicator of efflorescence.
The calcium carbonate begins to react with CO2 and moisture to form calcium bicarbonate. Converting the insoluble calcium carbonate to soluble calcium bicarbonate is slower than changing soluble calcium hydroxide to calcium carbonate. Therefore, calcium carbonate accumulates on the surface as a familiar white powder. In other words:
- calcium oxide + water = calcium hydroxide
- calcium hydroxide + carbon dioxide = calcium carbonate + water
- calcium carbonate + carbon dioxide + water = calcium bicarbonate
Calcium carbonate, followed by sodium sulfate and potassium sulfate, is the most common efflorescence salt compound. After concrete is poured, mortar is laid, or plaster is applied, the hydration (i.e. hardening) process begins, and calcium hydroxide is formed.
When does efflorescence occur?
The occurrence of efflorescence is not always predictable. The soluble salt components may be present, but instead of moving to the surface, they may be carried into the wall by any form of moisture or be absorbed by groundwater.
When weather is warm or dry and humidity is low, moisture usually evaporates before it reaches the surface, leaving salt deposits internally. Conversely, when weather is wet and cold and humidity is high, evaporation can be slow (or non-existent), with conditions ripe for visible efflorescence. As a building becomes enclosed during construction, efflorescence usually disappears.
Efflorescence can occur in manufactured materials within the first 48 hours of creation or installation. It is generally associated with the initial cure during manufacture of stone, cement, and brick masonry units and construction. This produces a construction-induced moisture that moves through the cementitious structure and picks up the free salts along the way. If the moisture makes it to the surface and evaporates there before it does so internally, white salt crystals form on the exterior surface. This is sometimes referred to as ‘primary efflorescence.’
Efflorescence also occurs when moisture is induced after construction from weather, landscaping, groundwater, or leaking pipes. Considering these possible sources, it can be surmised this ‘secondary efflorescence’ tends to occur randomly—it may affect some units or portions of the structure, but not others. The chemistry and formation of both are the same; the difference is when they occur.
 [5]
[5]Efflorescence is sometimes called ‘new building bloom’ because it frequently appears on ‘fresh’ buildings. It can occur during, or shortly after, construction and either disappear after a brief stay or remain for a year or more. Often confused with mortar scraping from a distance, it can occur during construction (Figure 7) or in the midst of cold, damp, or rainy weather. These conditions are usually temporary and suggest the building is drying out. Cold concrete and ambient temperatures encourage efflorescence because the salts are more easily dissolved, and concrete tends to bleed more in cool weather.
Contrary to what might be expected, wet-curing concrete tends to reduce efflorescence even though more water is introduced. When concrete is kept moist during a wet cure, additional capillaries and pores are filled to form a denser matrix that resists moisture migration. Concrete with a higher slump or not properly cured often has more open pores and capillaries to promote moisture migration in all directions.
Efflorescence is least likely to occur on the south and west walls because the warm sun moves the evaporation point from the surface to deeper in the wall. Walls facing north and east tend to experience the most efflorescence because they are usually cooler, permitting the evaporation point to remain on the surface.
Any type of moisture, including humidity, supports formation of efflorescence. Consequently, it tends to increase after a rainy winter, decreases with warmer spring weather, and virtually disappears in summer. When weather is warmer or dryer and humidity is low, moisture usually evaporates before it reaches the surface, leaving salt deposits internally. Conversely, when weather is wet and cold and humidity is high, evaporation can be slow to nonexistent and conditions are ripe. When efflorescence persists, there are internal problems and an investigation should be considered.
Until the structure is dry, efflorescence occurs even on interior walls (Figure 8). Drying usually happens from the inside out, and efflorescence can occur on both sides of a wall until the drying is complete. The dark moisture spots and white efflorescence suggest that the building is drying out. It usually disappears after the building dries and ambient conditions stabilize—otherwise, there is probably more to be concerned about than efflorescence. It may be signaling something is amiss, such as persistent moisture intrusion, or inadequate drainage or drying.
 [6]
[6]Photo courtesy Prosoco and Brick Industry Association
In many instances, brick’s tendency to effloresce can be determined prior to laying. To conduct this test, a brick is placed on end in a pan of distilled water for seven days. Water is allowed to migrate upward through the brick and evaporate on the surface. If the brick is prone to efflorescence, soluble salts will be deposited on its surface during evaporation. Distilled water ensures additional impurities are not introduced into the brick from the water.
Sources of salt and moisture
Examples of moisture sources include:
- construction activities (e.g. concreting, mixing mortar, plastering, fireproofing, and drywall installation);
- exterior sources (e.g. weather [rain, fog, condensation, humidity, and snow], landscaping irrigation, and groundwater);
- interior sources (e.g. cooking, showering, breathing, plumbing failures, drainage failures, missing or damaged vapor retarders, groundwater through the foundation, and failed or missing internal flashing); and
- installation problems (e.g. improper use of through-wall flashing, lack of sufficient weep holes, masonry without vented cavity, use of incorrect brick below-grade or at planter-type areas without a proper moisture barrier, failure of joint materials, or no integral water repellent in mortar or CMU).
The salts usually associated with efflorescence are alkali sulfates such as sodium and potassium. Sources of these salts include portland cement, lime, sand (source may be from sea shore), clay used in the brick (may come from saline earth), and any of the admixture containing chlorine. Even water that was in contact with sulfate-containing soil can produce efflorescence.
Efflorescence is also affected by the permeability of construction materials. The more porous the materials, the more they can transport moisture. Thus, smooth brick are less porous than textured brick, tooled mortar joints are less porous than untooled mortar, and standard-weight CMU is less porous than lightweight. Similarly, regular CMU is less porous than split-face, concrete with a steel trowel finish is less porous than a float finish, and architectural precast concrete panels with 41,368.5 to 48,263 kPa (6000 to 7000 psi) compressive strength are less permeable than 20,684-kPa (3000-psi) concrete.
Proper tooling masonry joints causes the cement paste to encapsulate the fine aggregate in a smooth dense matrix, forming a water-resistant joint. When masonry is pressure-washed, the chemical cleaner can damage or remove the cement matrix along with the joint’s water resistance.
Rain can easily enter a masonry wall that is under construction through its exposed top. Water can fill the cells in the brick and CMU units and then irrigate the walls long after completion. If the walls are reinforced, the pools of water in the cells can affect grout pours.
Removing efflorescence
Cleaning efflorescence only removes the visible symptoms—it does not necessarily cure the disease. So long as the causes are still present, efflorescence will keep reappearing.
Soft dry brush
If the salts are water-soluble, a dry brush with bristles stiff enough to remove the efflorescence, but not stiff enough to damage the surface of the substrate, may be used. Brushing actually removes the salts and prevents them from being driven back into the structure, as is often the case with fresh water and chemical treatment. This should be the preferred method because there is no detrimental effect on the surfaces.
Fresh water
Washing with fresh water is the next-best method. However, since moisture is one of the factors leading to efflorescence, washing also introduces more moisture into the wall. The paths the salts take to the surface are not one-way; some of the partially dissolved salts may be carried back into the structure through the same path that took them to the surface.
Manual washing can often draw additional salts to the surface. Repeat washing may be necessary, but when all the salts have come to the surface naturally and have been washed off, there will usually be no more trouble from this cause. It is imperative all efflorescence mechanisms are reduced or eliminated before sealing. If efflorescence does not return, chances are the moisture and/or salts were construction-induced.
Chemical
Probably the most common removal method, chemical cleaners can contain acids or detergents that dissolve the salts so they can be washed away with fresh water. The acids in the cleaner can react with pigments potentially in brick, concrete, or mortar, producing color variations.
Often, chemical cleaning cannot remove crystalline efflorescence because of the crystals’ attachment to the surface. This is why surfaces cleaned with chemical cleaning methods can look great for a few hours, but deposits are still visible once the surfaces dry. Contrary to what some may think, these are not new deposits emerging—they were existing ones temporarily disguised by the darkening effect of the initial treatment. The reappearing efflorescence is usually crystalline and is bonded to the surface. These deposits will ‘fizz’ on contact with strong acids (e.g. muriatic), which should not be used to clean masonry.
Chemical cleaning can produce acid burn if not properly performed by trained professionals (Figure 9). Although not considered efflorescence, muriatic acid frequently causes this burn; the brick’s porosity causes the acid to be absorbed before it can be properly rinsed. Improper chemical cleaning can also activate any metallic salts that may be dormant in the structure, resulting in stubborn brown and green stains instead of a stain-free structure. Therefore, these stains are not normally visible until after chemical cleaning.
 [7]
[7]Photo courtesy Joseph Crissinger
Proper pre-wetting and then rinsing are imperative. Chemical cleaning often produces stains from the previously mentioned metallic salts. Lime (white) scum can be a by-product of chemical cleaning and is usually caused when the masonry units are not adequately pre-wetted during washing. The strong cleaning acids can dissolve some of the portland cement matrix, which is then absorbed by the dry masonry and runs down the joints and walls to form a thin white film scum (Figure 10).
Chemical cleaners are usually formulated for certain types of salts and substrates. Some formulations may be for common alkali salts (e.g. calcium carbonate, sodium sulfate, and potassium sulfate) producing the usual whitish/grayish appearance. Other cleaners may be formulated for metallic salts from vanadium, manganese, and molybdenum.
Cleaners used for metallic stains can be more aggressive than those for alkali stains, and there is a risk of damaging or discoloring the brick. Product data sheets usually state what type of salt, and what color of masonry units, are compatible—the wrong cleaner can destroy the portland cement matrix of precast stone.
Before chemical cleaners are used, all loose efflorescence should be removed according to the previously mentioned soft brush removal procedure. Chemical removal must be done carefully and the cleaner and procedures should be tested in an inconspicuous area before proceeding. Pre-testing should not be performed on surfaces other than those being cleaned. This is because other surfaces may contain different salts or the substrate may be of another composition and produce different results than when used on the actual substrate to be cleaned.
Some removers may contain chlorine, which is also a salt and may contribute to the efflorescence problem. The cleaning instructions usually state to flush with plenty of fresh water, putting moisture (and possibly some of the partially dissolved salts) back in the wall, thus creating an efflorescence loop. It is best to minimize the amount of cleaner used to better control the amount of fresh flush water.
Mechanical
Mechanical methods, such as brush-blasting, definitely rid the efflorescence, but they are the most severe. These methods can also take off some of the substrate along with the efflorescence, roughen the surface (making it more porous to trap and hold moisture), and remove mortar matrix around the fine aggregate, lowering the mortar’s water repellency and leading to spalling.
Controlling efflorescence
Simply put, cementitious construction experiences efflorescence because it is constructed of natural materials. Ultimately, it is best to execute all available precautions to prevent it from occurring. However, one cannot have all walls facing south or west, or locate all buildings in the desert.
Efflorescence may not be completely eliminated, but there are steps that can be taken during design and construction to reduce the possibility of its occurrence. The suggestions listed in “Best Practices for Design and Construction[8]” include some of the most common and easiest to implement.
Sealers
Generally, sealers are either film-formers or penetrating (pore-plugging). Just as a lid keeps sauce in a jar, the former creates a thin layer over the substrate’s surface to hold in the salts, stopping salt-laden moisture just below the surface. When this happens, moisture usually evaporates and deposits the salts behind the sealer where they crystallize.
During crystallization, the salts expand and can cause brick to spall or deteriorate. On the positive side, film-formers can prevent moisture from entering the substrate from the exterior. Located on the surface, they are exposed to weather, ultraviolet (UV) light, dirt, and abrasion that shorten their life; they may also form a vapor barrier preventing the structure from drying to the exterior.
Some sealers are formulated to penetrate the substrate and plug the pores to stop the salts from ever reaching the surface. They are usually breathable, allowing the structure to breathe without carrying salt deposits to the surface. In cold-weather climates, freeze-thaw cycles can cause moisture trapped below the surface to expand and spall, or otherwise damage the masonry or joint.
Conclusion
As long as there is moisture, there will be efflorescence. Still, there is no definitive answer as to how long efflorescence will last—its lifespan is tied to weather conditions, moisture induced during construction, and design details. To some observers, it just seems to suddenly appear and then disappear; in reality, it can show itself any time from three weeks to more than a half-year after construction is complete.
It has been said efflorescence is controllable—not eliminable—and should not be a problem in modern masonry. Walls are going to get wet unless there is a giant umbrella over the structure. Cementitious materials come in various levels of textures and finishes and usually have some degree of porosity that is a good source of capillary action to transport moisture. This leaves the alkali salts. Considering the various salt sources, it is virtually impossible to ensure buildings remain on a ‘salt-free diet.’ The various salts can be effectively reduced, but not eliminated. Efflorescence, therefore, can be present, but invisible, lying in a dormant state just waiting for the right conditions to grace a building’s face.
Joseph “Cris” Crissinger, CSI, CCS, CCCA, ASQ, has almost 30 years of experience preparing construction specifications. As director of corporate specifications with McMillan Pazdan Smith Architecture (Spartanburg, South Carolina), he is responsible for evaluating new products, maintaining corporate masters, preparing project specifications, assisting in facility assessments, performing field investigations, and coordinating internal training programs. Crissinger is a member of Construction Specifications Institute (CSI), along with the Building Performance Committee of ASTM International, and the Design and Construction Division of the American Society for Quality (ASQ). He can be contacted via e-mail at ccrissinger@mcmillanpazdansmith.com.
- [Image]: http://www.constructionspecifier.com/wp-content/uploads/2012/02/Efflorescence_ExteriorBrickPartition.jpg
- [Image]: http://www.constructionspecifier.com/wp-content/uploads/2012/11/eff_Fig.-1-Efflorescence.jpg
- [Image]: http://www.constructionspecifier.com/wp-content/uploads/2012/11/eff_Figure-2.jpg
- [Image]: http://www.constructionspecifier.com/wp-content/uploads/2012/11/eff_Figure-7.jpg
- [Image]: http://www.constructionspecifier.com/wp-content/uploads/2012/11/eff_Figure-8.jpg
- [Image]: http://www.constructionspecifier.com/wp-content/uploads/2012/11/eff_Figure-9.jpg
- [Image]: http://www.constructionspecifier.com/wp-content/uploads/2012/11/eff_Figure-10.jpg
- Best Practices for Design and Construction: http://www.constructionspecifier.com/best-practices-f…and-construction/
Source URL: https://www.constructionspecifier.com/why-red-brick-turns-white-understanding-efflorescence/