Zinc-coated steel for the built environment: What architects and specifiers should know
by Samantha Ashenhurst | August 16, 2018 9:38 am
 [1]
[1]by Gary W. Dallin, P.Eng., and Frank E. Goodwin
Galvanized sheet is used in many industries, including construction, automotive, appliance, electrical hardware, drainage, and HVAC. While there is much information about the corrosion rate of zinc in the myriad environments where this product is used, the specified zinc coating mass is sometimes not suited for the end use. (For more, refer to Corrosion and Electrochemistry of Zinc by X.G. Zhang [Plenum Press, 1996] and Corrosion Resistance of Zinc and Zinc Alloys by F.C. Porter [Marcel Dekker, Inc., 1994].) This may be because corrosion principles are not understood, or a low coating mass is ordered to reduce purchase costs. Early failure can result if coatings are too thin for the application.
Architects and designers of products made from galvanized sheet must know the corrosion severity of the service environment, the design life of the product, and what constitutes a failure to wisely decide on the required zinc coating mass. Questions to be answered include:
- Is it an outdoor or indoor environment?
- Will the product be exposed or sheltered from the weather?
- What are the expected times/duration of wetness?
- Is exposure to chlorides, sulfates, and nitrates involved?
- Are local microclimates (e.g. heavy industry) a factor?
- For indoor applications, will the environment be controlled?
- In the case of buildings, is animal confinement, an indoor pool, or some other moisture/high-humidity source involved?
- Do all parties understand the expected service life and what will be considered a failure (e.g. first appearance of red rust or perforation of the steel substrate)?
- What will be the consequences of failure?
Once all questions have been answered, the corrosion rate of the zinc can be estimated. Combined with the required design life, the minimum coating mass needed can be calculated. For example, design life of 40 years multiplied by corrosion rate of 0.5 microns (μm) per year equals a required coating thickness of 20 μm.
Determining the required coating mass for a given product is therefore not generally a difficult task since the service life of a galvanized coating is a linear function of the environment in which it is placed. However, due to cost reduction requests from users of galvanized sheet, there is growing demand to produce thinner zinc coatings. (For more, refer to “Review on Wiping: A Key Process Limiting CGL Productivity,” by F.E. Goodwin and M. Dubois, presented at the June 2011 Galvatech conference in Genoa, Italy.) This is sometimes at odds with the capability of zinc to meet the service life needed for the intended environment. While many products do not need thick zinc coatings due to benign service conditions or short one-time usage, some thin zinc coatings are applied on end products where early failure is guaranteed. Such applications are a waste of steel substrate, which would have a much longer service life if applied with a thicker zinc coating. It is quite likely false economy, as it will not minimize life-cycle costs (i.e. an alternative with a higher initial cost may be economically justified by reductions in future costs). (For more, refer ASTM A903-09, Standard Practice for Life-Cycle Cost Analysis of Corrugated Metal Pipe Used for Culverts, Storm Sewers, and Other Buried Conduits.)
Continuously galvanized steel sheet is produced on coating lines using gas wiping to remove the excess liquid zinc drawn up from the molten bath by the moving strip. Gas wiping is an efficient process that has been used for many decades and is now at the stage where it can closely control the final coating thickness at line speeds from 50 to 200 m (164 to 656 ft)/min with low maintenance and reasonable investment costs. To produce thinner coatings, though, gas pressure must be increased, and the gas knives moved closer to the moving strip, which can rapidly bring the process near its operational limits. In these cases, line speed must be reduced to achieve a thinner coating. Even then, each coating line has a zinc thickness below which it is physically not possible to wipe. Needing to run slower than rated capacity lowers line productivity and increases operational costs. (For more, read the paper, “Challenges in the Production of Thin Coatings at High Line Speed,” by M. Dubois, et al, presented at the November 2009 Asia–Pacific Galvanizing Conference in Jeju, Korea.) Purchasers expect thinner coatings will reduce the product price, but in fact, the production cost may increase.
The optimal coating mass for a particular product made from galvanized sheet is therefore a balance between the required service life, and the ability of a zinc coating line to produce coating mass at a reasonable cost. If a thin coating is the right product for a particular end use, then the selling price may have to be higher to offset lower coating line productivity, or it may be cheaper to apply more zinc than required. These considerations must be taken into account by the buyer.
 [2]
[2]Images courtesy The International Zinc Association (IZA) – GalvInfo Center
Coating thickness designation systems
Most global standards for galvanized sheet, including ASTM International, do not directly use thickness to specify zinc coatings. It is very difficult and expensive to directly and accurately measure the thickness of zinc on coated sheet, as the coating is usually a small fraction of the total coated sheet thickness. In the case of a single coil of galvanized sheet, if thickness is measured manually (and even if accurately done), it is but one very small point on a large surface area, with no assurance the result is representative of the overall coil. A more representative method is to use a destructive, weigh-strip-weigh technique to measure all the zinc on a larger surface area of sheet. This is the main reason galvanized sheet standards specify the coating in terms of mass (weight) per unit area.
The industry does use expensive X-ray and radioisotope fluorescence devices, both on- and off-line, for directly sensing zinc thickness. This equipment requires considerable expertise to operate. On-line units continuously measure the coating thickness as the strip passes by. However, they must regularly be calibrated via the weigh-strip-weigh technique. Further, the real-time results provided are mathematically converted to mass per unit area for purposes of assessing compliance with ASTM and other specifications.
Service life data
In the atmosphere, the corrosion rate of a zinc coating varies widely depending on many environmental factors. For example, “time of wetness” is an important issue affecting corrosion rate (e.g. outdoor applications in a dry climate like Dubai are very different from locations experiencing high annual rainfall or extended foggy periods). Also, the presence of impurities such as sulfates, chlorides, and nitrates can dramatically affect corrosion rate. The presence of these compounds is quite often a function of localized microclimates (e.g. heavy industry and salt spray from major roadways or a sea coast).
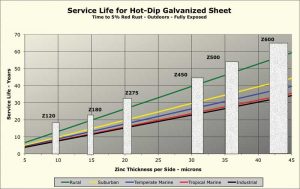
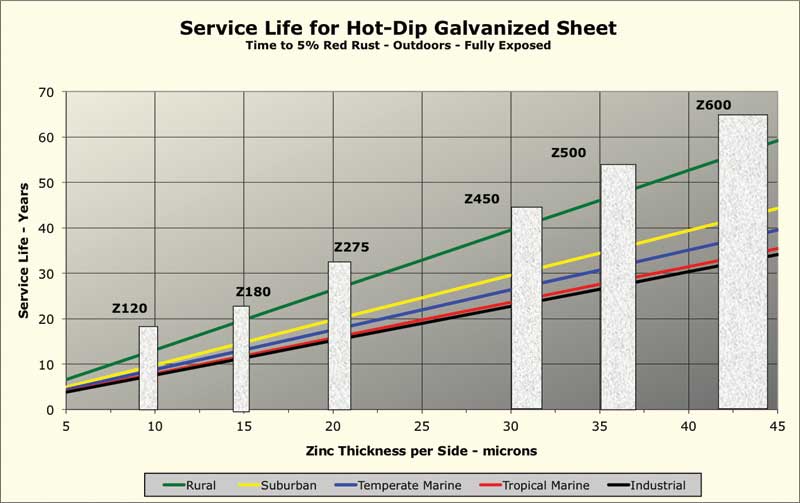
Figure 1 shows calculated corrosion rates for six different North American cities in each of the five climate categories. The corrosion rates shown are estimates based on models using data from environmental databases. The calculated corrosion rates used to generate this chart are averages for the six cities. Six common coating mass “bars,” per ASTM A653/A653M, Standard Specification for Steel Sheet, Zinc-Coated (Galvanized) or Zinc-Iron Alloy-Coated (Galvannealed) by the Hot-Dip Process, have been overlaid on the chart. To determine the corrosion rate for a specific locale, the documented actual environmental data can be entered into the software. (For more, click here[3].)
Although the corrosion rate can vary considerably depending on local environmental factors, as Figure 1 indicates, the life of a zinc coating is a linear function of coating mass for any specific environment. As is also seen in Figure 1, when the per-side coating thickness is much below 10 mm, outdoor service life is less than 10 years, making the use of such thin coatings outdoors an unwise choice.
Note the zinc corrosion data given in Figure 1 is from after circa 1975, when aggressive pollutants such as sulfur dioxide began to decline from their higher levels of the mid-20th century. The service life of galvanized sheet in an urban industrial area is now longer than it was 40 to 60 years ago. On the other hand, corrosion rates in marine environments have not changed much since the rate of zinc loss is governed more by the amount of deposited sea salt than airborne pollutants.
Design vis-à-vis service conditions
“Mistakes in plant design are the most frequently cited (58 percent) cause of corrosion failure in chemical-process industries.” (Corrosion Control by S.A. Bradford, published in 1993 by Van Nostrand Reinhold International [UK].)
While this quote does not refer directly to products manufactured with galvanized sheet, design plays just as important a role in their corrosion failures.
 [4]
[4]Photos © BigStockPhoto.com
A number of general principles are necessary for a successful design. Those most applicable to products made with galvanized sheet are:
- minimize attack time;
- restrict galvanic cells;
- protect against environmental cells; and
- design for inspection and maintenance.
As mentioned earlier, zinc coatings corrode uniformly at known rates in the atmosphere, and most other environments, so the coating thickness needed to last for the design life of an application can easily be calculated. The best designs take advantage of the uniform corrosion of zinc, avoiding situations causing accelerated, localized attacks.
When zinc is exposed to wet/dry weather cycles it forms a very thin, protective zinc oxycarbonate patina. (For more, click here[5].) However, a very small amount of zinc is dissolved by rainwater and removed during each wetting event. In designing a structure, it is important to ensure the entire exposed surface (e.g. roof elevation and side wall) always “sees” the same weather as uniformly as possible across the entire surface area. If some portion of a roof receives extra water, draining from some higher, non-zinc coated surface, then the extra water takes its share of zinc (in addition to the amount taken by the rain that fell directly on it). The area of impingement has its zinc coating removed at a higher rate.
In designing a building subject to weather, avoid configurations where water drains incompletely and/or pools. When subjected to long periods of wetness, the protective zinc oxycarbonate patina layer breaks down, increasing the zinc corrosion rate. Well-designed structures clad with galvanized sheet have good drainage and dry uniformly and quickly after a rain event.
Some soils are very corrosive to zinc. To avoid problems, design galvanized sheet on buildings away from the ground.
Galvanized sheet used to clad some commercial and industrial buildings, and many farm buildings, is sometimes the only separation between the inside and outside “climate” (i.e. there is no inner wall or ceiling structures separated from the outer wall by insulation). This is the case in many animal confinement buildings, where it can become warm and humid inside. In the winter, condensation can form on the inside of the wall and roof panels. If ventilation is poor, condensate can be present for long periods, accelerating corrosion of zinc coatings. Animal waste decomposition products (e.g. sulfides, ureas, and amines) become dissolved in the condensate, adding to the corrosivity of the environment. (For more, refer to the U.S. Steel technical bulletin TBP 2005.2, “The use of steel building panels for animal confinement.”) The solution is to have the building design include insulation with adequate ventilation to both remove gaseous decomposition products and prevent moisture buildup and condensation inside the structure.
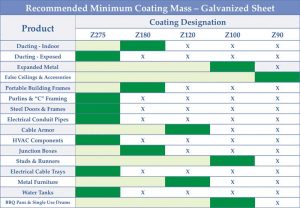
Sometimes, even with the right coating mass, problems can arise. Using a coating thinner than required to save some material costs upfront can only compound design errors, bringing on an even earlier failure. Figure 2 shows GalvInfo Center’s recommended minimum coating mass to be used for a variety of common galvanized sheet applications. (For more, click here[6].)
 [11]
[11]Gas wiping process
The gas wiping process used to apply zinc coatings on continuous coating lines has many practical advantages. It is simple, requires minimal maintenance, has a low operational cost and low energy requirements, and a large process window. The sheet vertically exits the bath at high speeds, dragging out more zinc than needed for the coating. Gas knives employ low-pressure/high-volume gas streams (almost always air) impinging against both sheet surfaces. The gas flows through two knife boxes positioned parallel and adjacent to each side of the strip. It escapes through precisely machined slot openings placed about 10 to 12 mm (395 to 470 mils) from the traveling strip. The gas jet acts as a knife, stripping the excess molten zinc and forcing it back down to the coating bath. For line speeds over 100 m/min and coating thicknesses below 20 μm, the final thickness depends linearly on the nozzle-to-strip distance when the distance is more than seven times the nozzle opening.
As a result of the limited effect of the distance when it is less than seven times the nozzle gap, wiping infinitely close will not lead to an infinitely thin coating.
The nozzle-to-strip distance cannot be kept safely below 7 to 8 mm (275 to 315 mils), since the risk is too high of the strip touching the nozzles due to strip vibration and/or not perfectly flat sheet.
When the nozzle-to-strip distance is less than six to seven times the nozzle opening, the coating thickness will decrease only slightly. At less than below this wiping distance it is generally accepted the risk of the knives touching the strip is too high, especially on wide material, and the minimum nozzle opening should be 0.8 mm (30 mils).
Thin coatings on thin sheet are expensive to produce. The present best per-side industrial practice is about 35 g/m2 (5 mm). (For more, consult “Maximum Line Speed Limit with a Minimum Coating Weight, Where is the Limit?” by M. Dubois and J. Callegari, presented at the June 2011 Galvatech conference in Genoa, Italy.) Using the best wiping practice, 50 g/m2 becomes cheaper than 35 g/m2 on thin sheets due to the needed reduction in line speed to reach thin layers. The minimum galvanizing cost is in the range of 52 to 72 g/m2/side.
As coatings on thin sheet are more expensive to produce, it is economical to give away extra zinc to increase line productivity. It is in the best interests of continuous galvanizing lines to develop a cost map for the particulars of each line. Marketing staff would then know the sheet thickness range of when it makes sense to offer a 35 g/m2 (5 mm) per side coating vs. when to decline such orders or add a surcharge to offset the reduced productivity.
There are also a number of quality issues associated with producing thin zinc coatings while running at high line speeds, such as bright spots and an edge effect due to accumulation of oxides.
The gas wiping process also has a physical limit related to a splashing phenomenon. The air knife can become inefficient, requiring an immediate line slow down to allow the exploding back flow to remain on the sheet.
Narrow nozzle openings are very sensitive to dust, zinc splashes, and scratches, all of which can lead to jet (blower) lines. In practice, an opening below 0.8 mm is very difficult to operate.
The amount of dross (floating zinc oxide [ZnO] scum) produced is also a large concern when producing thin coatings at high line speed. When total gas flow from the knives is too high, the level of dross becomes unacceptable. In the case of zinc containing 0.25 percent aluminum run at 130 m/min it was observed the dross created was 25 to 30 g/m2 for both sides. At 80 m/min the amount of dross dropped to 12 to 15 g/m2. Dross must be removed during top drossing of the bath to avoid entrainment in the coating and surface defects on the final product.
Pre-painted galvanized sheet
Coil-coated steel sheets are widely used in construction, automotive, and appliance industries. Their superior corrosion stability is explained by the synergistic protection provided by metallic and organic coatings. (Note: For more, read “Effect of steel and coating thickness on edge creep of coil coated materials” by T. Prosek, et al. presented at the 2015 Galvatech conference in Toronto, Canada.) Besides defects such as scratches occurring during transport, processing, installation, and product service life, the cut edges of pre-painted panels expose the steel and are usually not treated. Paint delamination at cut edges is a principle reason for the failure of coil-coated steel sheets.
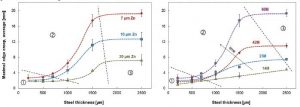
The aim of the study in reference was to investigate the role of the metallic coating thickness on long-term cut edge protection of coil-coated products. (See previous note.) Paint delamination from cut edges of coil-coated materials was followed as a function of exposure time at a marine test site for 17 hot-dip galvanized materials differing in the thickness of zinc coating (7 to 20 μm) and steel substrate (0.2 to 2.5 mm [8 to 100 mils]). Results are shown in Figure 3.
 [12]
[12]The study concluded edge creep (i.e. corrosion under the paint creeping in from cut edges) increases with decreasing zinc thickness and increasing steel thickness. For steel substrates over 0.75 mm (30 mils), any reduction in coating thickness below Z275 (20 μm) leads to an accelerating drop in service life due to edge creep. (Note: The “Z” means the coating is galvanized [zinc] and the number refers to the mass of zinc on the surface of the steel sheet in g/m2 [e.g. Z275 = 275 g/m2 total both sides]). For steel thickness less than 0.75 mm, the effect of zinc thickness in protecting against edge creep was not so pronounced.
In the case of pre-painted galvanized sheet, zinc supports the paint, protecting against under-film corrosion. The thicker the zinc the slower the under-film corrosion proceeds, so the protection time is longer. This is even more the case as the steel substrate thickness decreases. As paint costs from five to 25 times more than Z275 coatings per unit area, using thinner zinc coatings in exposed environments is an ineffective and wasteful use of resources, particularly in cases where the steel thickness is above 0.75 mm. Once the zinc layer is gone, the paint is of little value in protecting the steel.
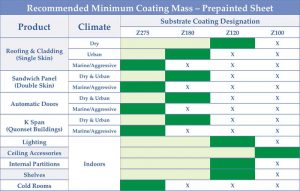
These field performance results indicate a Z275 coating is strongly advised for all exterior pre-painted sheet applications, except for the potential use of Z180 in cases where the climate is dry and the environment no more aggressive than urban.
Figure 4 shows recommended minimum coating mass to be used for a variety of common pre-painted galvanized sheet applications.
Conclusion
The coating thickness of galvanized sheet determines its life. To select the proper coating mass, it is important to know and understand the following:
- the expected service life of the end use;
- what defines a failure;
- the particulars of the service environment and the coating mass needed to meet the service life;
- selection of coating designations too thin for the application will result in reduced corrosion performance of the product (in an almost linear relationship);
- the coating mass having the minimum production cost is somewhere in the range of 52 to 72 g/m2/side for zinc coatings; and
- for pre-painted sheet, the importance of enough zinc being present on the substrate to minimize under-film corrosion and outlast the expensive paint coating layers.
When deciding on the required coating mass there are a number of considerations. Designers should use the wealth of published knowledge on the corrosion rate of zinc in most environments. When this is not taken into account, or ignored, it is false economy to sacrifice service life to save the relatively small upfront zinc cost of a too-thin coating mass. Certainly, in the authors’ experience using less than 120 g/m2 for outdoor applications is unwise, both from a product life-cycle cost and a sustainability standpoint. Ordering thinner coatings decreases coating productivity and increases costs, as producers may ask a higher selling price. Also, thin, hot-dip zinc coatings can sometimes be impossible to produce. In the case of pre-painted galvanized sheet, zinc supports the paint, protecting against under-film corrosion, so the zinc coating should be thick enough to outlast the life of the paint cover.
Aside from the steel substrate, zinc is the single largest cost component of galvanized sheet. It is understandable why the marketplace wants to use thinner zinc coatings. Architects and specifiers can use this article to better understand how service environment, using the correct coating mass, and proper design can minimize corrosion, lengthen service life, and reduce life-cycle costs.
Gary Dallin, P.Eng., is director of the GalvInfo Center, a program of the International Zinc Association (IZA). Previously, he was employed as a metallurgist in the Canadian steel industry. Dallin has 48 years of experience with galvanized and pre-painted steel sheet products, is a registered Professional Engineer in Ontario, and is active on ASTM International Committee A05 on metallic-coated steel products. Dallin can be contacted at info@galvinfo.com[13].
Frank E. Goodwin is executive vice-president of International Lead Zinc Research Organization (ILZRO) and also director of technology and market development of International Zinc Association (IZA). Goodwin joined ILZRO in 1982 as manager of program development after serving as assistant director of product and process development at Chromalloy Research and Technology Division in Orangeburg, New York. Following his appointment as ILZRO’s manager of metallurgy and program development in 1984, he was promoted to vice-president of materials science in 1986. He holds a master’s degree and a PhD in materials engineering from the Massachusetts Institute of Technology in Cambridge, Massachusetts. Goodwin is also the author of several national and foreign patents as well as numerous articles and book contributions. He can be reached at fgoodwin@zinc.org[14].
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2018/08/bigstock-165530555.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2018/08/Zinc.jpg
- here: https://www.galvinfo.com/wp-content/uploads/sites/8/2017/05/GalvInfoNote_1_6.pdf
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2018/08/bigstock-218943667.jpg
- here: https://www.galvinfo.com/wp-content/uploads/sites/8/2017/05/GalvInfoNote_3_1.pdf
- here: https://www.galvinfo.com/wp-content/uploads/sites/8/2017/05/GalvInfoNote_2_5.pdf
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2018/08/Figure-1.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2018/08/Figure-2.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2018/08/Figure-5.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2018/08/Figure-6.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2018/08/bigstock-Steel-Building-garage-240515.jpg
- [Image]: https://www.constructionspecifier.com/wp-content/uploads/2018/08/bigstock-191523901.jpg
- info@galvinfo.com: mailto:info@galvinfo.com
- fgoodwin@zinc.org: mailto:fgoodwin@zinc.org
Source URL: https://www.constructionspecifier.com/zinc-coated-steel-for-the-built-environment-what-architects-and-specifiers-should-know/
 [7]
[7]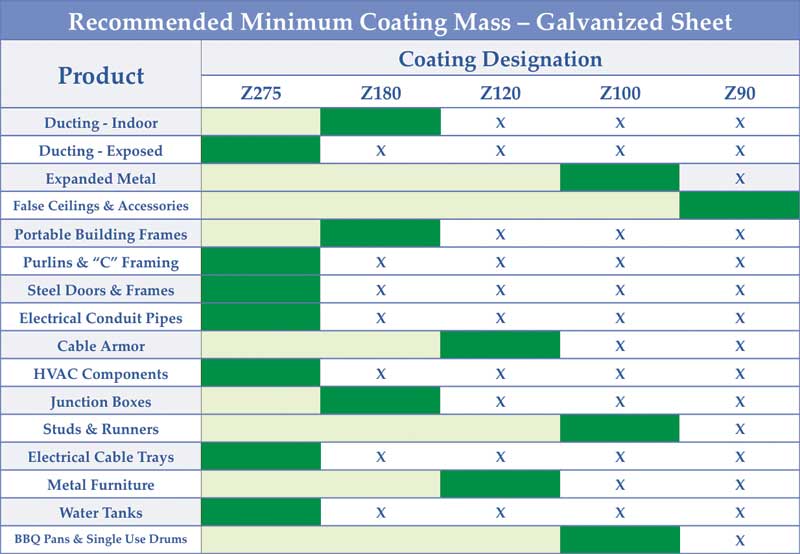 [8]
[8]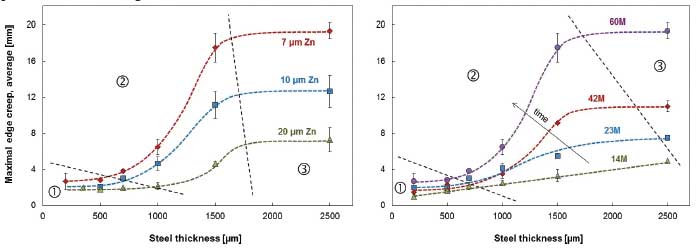 [9]
[9]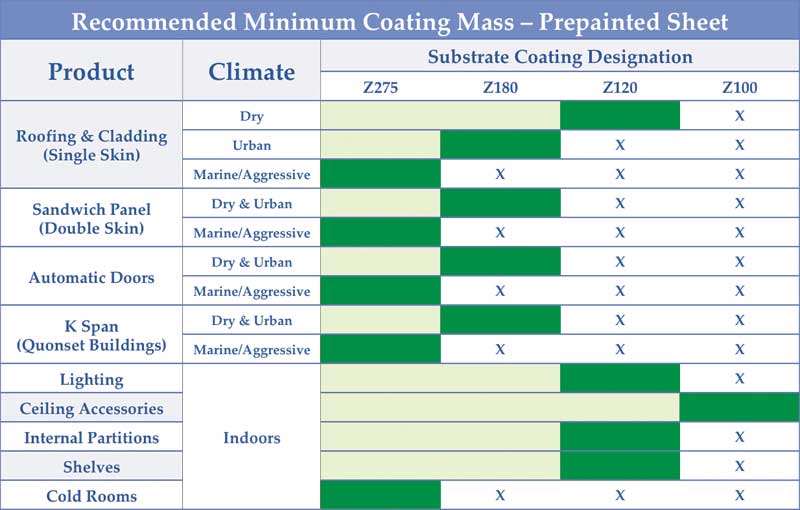 [10]
[10]